Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2015) Volume 4, Issue 3
This work describes the chemical characterization of Aloe vera leaves after dissecting the whole leaf into filets and skin, and characterizes the mucilaginous gel extracted from the filets. Gel was extracted by hand-fileting the leaves and pressing the resulting filet. The mean gel yield was 86.3% from the whole filet and the volume of freeze-dried gel was 0.91% that of fresh gel. The volume of the pressed fresh residue (cake) of the filet after extracting the gel was 15.1% that of the whole filet. The fresh gel had a moisture content of 97.8%, a pH of 5.24 and 0.07% acidity expressed as malic acid. Water content analysis of the freeze-dried gel and dehydrated cake revealed moisture values of 2.3% in the gel and 6.9% in the cake. The ash content was 17.20% in the gel and 13.15% in the cake. The total dietary fiber determined in the cake (71.5%) was greater than that of freeze-dried gel (64.8%), and water retention capacity (WRC), swelling (SW) and fat adsorption capacity (FAQ) were higher in the cake than in the gel. Aloin and polysaccharides were also determined in both the freeze-dried gel and cake. Antioxidant activity was greater in freeze-dried gel (46.7%) than in fresh gel (36.7%). This study aims to analyze the process by which gel and latex is extracted from aloe vera grown and utilized in Chile, comparing it with other Aloe barbadensis varieties cultivated elsewhere.
Keywords: Aloe vera; Gel powder; Polysaccharides; Antioxidant activity; Phytochemical analysis
Aloe vera (Aloe barbadensis Miller) is a perennial monocot plant with turgid green leaves joined to the stem in a rosette pattern. Aloe leaves consist of a thick epidermis (skin) covered by a cuticle surrounding the mesophyll that includes chlorenchyma cells and thinner walled cells that form the parenchyma (filet). The mesophyll cells contain a transparent mucilaginous jelly called Aloe vera gel [1].
Aloe vera is native to western meridional Africa and belongs to the group of crassulacean acid metabolism plants (CAM plants) with nocturnal CO2 assimilation, which prevents water loss in hours when there is less evaporative demand. The species is therefore adapted to arid and semi-arid regions such as northern Chile (from the II to IV Regions) [2]. These regions have an arid Mediterranean climate with mean annual precipitation of 170 mm in rainy years and 40 mm in dry years. The mean maximum temperature is 32°C at midday in summer, with occasional peaks of up to 45°C. The average minimum night time temperature in winter and spring is 6°C [2,3].
Aloe vera leaves and leaf gel have been used commercially since ancient times. Their beneficial medicinal, cosmetic and nutritional effects have been described, but until recently no experimental scientific evidence existed validating them. However, many accounts describe how the gel induces rapid healing of wounds and stimulates macrophages in the immune system. It is also known that the anthraquinones in the leaves can act as laxatives and as antifungal and antibacterial drugs [4-6]. Today it is accepted that the medicinal, cosmetics and nutritional properties of Aloe vera are due to the molecules the plant synthesizes. Two classes of polysaccharides in the gels and leaves work to prevent the development of colon-rectal cancer and induce effective wound healing. Both properties are currently under investigation by our group.
The chemical composition of plant constituents depends on geographical distribution, soil quality, water availability, solar radiation and temperature. Water availability is important, since the physiological role of leaf gel is to retain water. The amount of water in the gel fluctuates between 98.5% and 99.5% of fresh weight, while more than 60% of the remaining gel consists of polysaccharides [1-3].
To process the leaf, its products are separated into gel and cortex. The latex is in the cortex and corresponds to the bitter yellow juice of the green part of the leaf produced in the leaf epidermis and in the spiny portion of the leaves. The bitter juice contains hydroxyanthracene derivatives (15-40%). Aloe gel is the colorless mucilage within the inner part of the fresh leaves [7] and consists primarily of water (>98%) and polysaccharides (pectins, cellulose, hemicellulose and an acetylated galactoglucomannan called acemannan). Acemannan is the main functional component of the gel, and is formed by a long chain of acetylated mannose intercepted with glucose, in which the mannose holds branches of galactose [1,8]. Aloe gel is commercialized as a concentrated powder and used as a wound-healing agent and immune system stimulant [9,10]. The physiological activity of Aloe vera polysaccharides as wound healing agents has been widely studied using in vitro cell cultures to determine the rate at which cells engaged in tissue repair such as fibroblasts and epidermal and endodermal cells proliferate [11,12].
This study aims to analyze the process by which gel and latex is extracted from aloe vera grown and utilized in Chile, comparing it with other Aloe barbadensis varieties cultivated elsewhere. We also analyze the dehydrated pressed solid residue (cake) – of interest due to its high dietetic fiber content and the physical and chemical parameters (pH, humidity, acidity, etc.) of each product obtained in this manner, as well as steroid, triterpene, flavonoid and aloin content, among other aspects.
Plant material and tissue separation
Aloe vera (Aloe barbadensis Miller) leaves were gathered in February, 2010 in Coquimbo in the IV Region of Chile, at a latitude of 31°S. Specimens were delivered to the Facultad de Ciencias, Universidad de Chile and plants were identified by Dr. Jose San Martin (University of Talca, Plant Biology and Biotechnology Institute) as Aloe barbadensis Miller.
Samples were kept frozen (-40ºC) and protected from light until the analytical procedure took place. The leaves used for extractions measured between 40 and 60 cm in length and were taken from 3-year old plants. Whole leaves were cleaned by washing them individually with distilled water and water with 0.5% chlorine. The spikes and margins were removed before slicing the leaf. The cortex was carefully separated from the parenchyma using a scalpel-shaped knife. Filets were washed thoroughly with distilled water to remove the exudate from surfaces. Fresh aloe filets were stored for no longer than 1 h at -18ºC prior to lyophilization.
Dehydration
The extracted gel was lyophilized in a Thermo Savant Model D-230 at -50°C under vacuum. The pressed cake, consisting of filet residues, was kept in a pressurized filter and dehydrated in a Heraeus TU 60/60 WC (Heraeus GMHB Hanau) oven at 80 ± 5°C. Both products were reduced to powder and stored at room temperature in closed glass flasks to prevent gel humidification.
General experimental procedures
Unless otherwise stated, all starting materials were obtained from commercial suppliers and used without further purification. Column chromatography took place using silica gel 60 (Merck, Darmstadt, Germany). Thin layer chromatography (TLC) was performed on silica gel GF254 (Merck) using two kinds of solvents: (1) CH2Cl2:MeOH (3:1), and (2) AcOEt–MeOH (4:1) and/or aluminum oxide 60 F254 (Merck) using CH2Cl2:MeOH (10:1) as a solvent. Spots were detected by UV light and Dragendorff or Liebermann–Burchard, anisaldehyde and NP/PEG reagents. Preparative TLC was performed on 2-mm thick silica gel F254 plates (Merck).
Analytical methods gel and cake analyses
Moisture: Water amounts in cake material (CM) and fresh gel were determined following the method described by the AOAC [13]. To do so, 3 to 5 g of duplicate samples were dried in an air oven at 103ºC for 5 hours. In some cases, samples were dried for an additional hour until the variation between two weights was a maximum of 5 mg. The following mathematical ratio was used
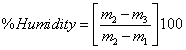
m1: mass of empty capsule
m2: mass of capsule+sample
m3: mass of capsule+dry sample
pH: pH was determined in fresh samples as indicated by the AOAC (AOAC, 2005), using a Hanna Instruments HI 8424 Microcomputer pHmeter.
Acidity: Acidity was determined by potentiometric titration with NaOH 0.1N with a pH of 8.1 ± 0.1 using phenolphthalein as a pH indicator as per the AOAC method [13].
In order to determine pH, 10 g of samples were placed in flasks, after which water was added to complete 100 mL. Results were expressed as percentage of malic acid.
Color: Color was determined using the procedure proposed by Loyola [14]. This methodology applied was originally used for determining the color of white wine, and measures the transmittance of wine at four wavelengths: 445, 495, 550 and 625 nm, to obtain three values (X, Y and Z) expressing the proportions of red, green and blue to reproduce the wine?s color. The x and y coordinates were calculated to represent color in a chromaticity diagram (CIE).
Antioxidant capacity: Antioxidant capacity was determined in triplicate samples, analyzing gel and pressed cake using the 2,2-diphenyl-1-picrylhydrazyl discoloration assay [15]. The extracts were subject to spectrophotometric free radical scavenging at 517 nm by residual absorbance (A) of the DPPH radical. A freshly prepared DPPH methanol solution (20 mg/l) was used for assays. Samples were dissolved in methanol. The degree of discoloration indicates free radical scavenging efficiency of samples. Quercetin (Aldrich, Buchs, Switzerland) was used as a reference for free radical scavenging. The DPPH discoloration percentage was calculated as follows:
Discoloration (%)=(1-Compound with DPPH-Blank sample)/ (DPPH control)×100.
Extracts were assayed at final concentrations of 400, 200, 100, 50, 10 and 1 μg/ml. EC50-DPPH is the concentration of antioxidants produced at 50% discoloration. In order to determine EC50-DPPH, each tube was wrapped with aluminum foil and kept in the dark at 30 °C for 30 min. Measurements were made under dim light.
Spectrophotometric determinations were made using a UNICAM spectrophotometer, and results are presented as a mean value ± SD [15].
Lyophilized gel and cake analyses
Moisture determination: (Dry weight) Samples were dried by heating them to 40 °C under normal pressure at constant weight, following the method proposed by AOAC [13]. The moisture % was calculated based on the difference in sample weight before and after dryness.
Proteins: Protein content was determined spectrophotometrically by the method proposed by Bradford, [16], using a 1 mg/ml solution of bovine seroalbumine as a standard protein. The protein percentage was calculated with the following formula [17].

Fiber determination: Total fiber and dietary fiber were determined in 2 g of a fat-free sample using the acid- base digestion method, in which gel and cake are macerated and placed in a flask, adding 300 mL of H2SO4 1.25% w/w. The mixture was heated under reflux for 30 min, occasionally rotating the flask. The solution was filtered under reduced pressure in a Büchner funnel. The residue was washed 3 times using 50 mL of boiling water to stop the acidic reaction and returned to the flask under reflux, adding 300 mL of 1.25% w/w boiling NaOH. The mixture was boiled for 30 min. and filtered again in a Büchner funnel. The residue was washed with 25 ml H2SO4, followed by 3 washes with 50 mL of boiling water and a final wash with 25 mL 95% ethanol. The residue was transferred to a crucible for oven drying at 130ºC ± 2ºC for two hours and finally incinerated for 30 min. at 600ºC ± 15ºC. The results were expressed as per the official method [13].
Ash determination: Ash content was gravimetrically determined by heating the samples to 550ºC overnight [13].
Determination of non-nitrogenous compounds
The non-nitrogenous extract consists of all molecules not determined by specific analytical methods. Carbohydrates, vitamins and non-nitrogenous soluble organic compounds are considered nonnitrogenous and were quantified by the indirect method as indicated in the official guidelines [13].
To do so, the non-nitrogenous extract % was determined by subtracting the moisture, fat, fiber, ashes and protein %.
%NNE =100 − (A + B + C + D + E)
Where, A=% of moisture; B=% protein; C=% ether extract; D=% fiber; E=% of ashes.
Anthraquinones: Anthraquinones were identified by thin layer chromatography (TLC) in silica G F250 using aloin as a standard [18,19], and determined by mixing 0.5 g of the sample with 2 mL methanol. The mixture was heated to 40ºC in a water bath and subjected to ultrasound for 15 min. It was then centrifuged for 5 min at 10000 g and the supernatant was analyzed by TLC.
Aloin and polysaccharides were also determined in both freezedried gel and cake.
Determination of Aloin percentage: Aloin content was determined in triplicate as per the methodology described in ref. [20,21]. The absorption spectrum was prepared using spectrophotometric scanning of a 10 ppm aqueous aloin solution with maximum absorption at 300 nm. The absorption curve was drawn from aloin solutions prepared at 5, 10, 15, 20, 25, 30 and 40 ppm. Aloin was quantified in lyophilized gel, in pressed cake and in the leaf epidermis. The standard concentration of the solution was 1000 ppm (1mg/1mL distilled water). Before determinations, aloin samples were filtered using 1.2 μm mesh glass fiber paper.
Polysaccharide determinations: Polysaccharide acemannan was quantified using the Congo Red colorimetric assay [22,23]. Its determination was based on the interaction of Congo Red with the glucomannan (acemannan) of the gel, which creates a permanent, red-colored complex with an intensity proportional to the amount of acemannan in the sample. For this procedure, the leaf filet was macerated and filtered using a cheese cloth in a vacuum to discard solid residues arising from cell walls. The macerate was then lyophilized. One g of lyophilized gel was dissolved in 200 mL of deionized water and agitated for three hours. The solution was filtered in a vacuum using 1.2 μm mesh glass fiber paper, and then lyophilized and stored in plastic tubes at room temperature. In order to prepare the standard calibration curve, 1 mg/mL of glucomannan P.A. dissolved in water (AMRO) was used as a standard. For measurements, 400 μL of the standard and samples were placed in tubes with 500 μL of 50% NaOH and gently agitated, adding 100 μL Congo Red 2x 10-4 M. The resulting mixture was allowed to stand for 20 minutes before taking a reading at 540 nm.
Chemical-physical properties of the gel and the pressured cake
Water retention capacity (WRC): WRC was determined in 0.25 g of each sample by adding 5mL of 1 M phosphate buffer at pH 6.3 and leaving it for 24 hrs, after which samples were centrifuged at 15000 rpm for 15 min. The pellet was dried overnight in an air-oven at 102ºC. WRC is expressed as per the ratio shown below
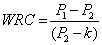
k=(P1-P2), a=28 x 10-3 g of phosphate/mL; P1=g of residue before dryness; P2=g of dry residue
Swelling (SW): In order to determine the gel’s swelling capacity , 0.25 to 0.30 g of gel samples were placed in graduated 15 mL conical tubes to measure initial gel volume. Ten mL of phosphate buffer was added to each tube. Samples were agitated and after 16 hours in the buffer, volume was determined again. The difference between the original and final volume was recorded. The increase in each sample’s volume was expressed as mL of gel per gram of dried sample [1].
Fat adsorption capacity (FAC): FAC is the maximum quantity of fat retained by the fiber present in the gel [24,25]. FAC was determined by mixing 0.25 g of gel with 30 mL sunflower oil. The mixture was left overnight at room temperature, after which it was centrifuged for 10 min at 15000 rpm, discarding the supernatant. The precipitate was weighed and the capacity for fat retention was expressed as g oil/g dry weight.
Yield and physical and chemical properties
Gel production yield was calculated considering the average weight of aloe vera leaves to be 100%. The leaf cortex represented 34.23% of the total, while the gel filet was 64.03%, with 1.74% loss due to handling. This yield does not differ significantly from previous reports of 35.8% cortex, 61.3% gel filet and 2.9% loss [16].
The yield from the manual pressing method for gel and cake was 45.58% and 52.02% of fresh filet, respectively, with 2.4% loss in the pressure filter. The percentage yield obtained for fresh gel was less compared with other reports in the literature [1,16]. However, the gel extraction methodology reported in those publications is different than that used here.
The pressure cake yield was greater than that found by [16], who reported 13.1% cake production with retention of 4.6% in the pressure filter. Lyophilized gel yield was 0.90% while pressed cake yield was 0.92%, confirming that most gel and cake is water. Water may take two forms in plant and animal tissues; “free” and “bound.” Free water is the common form of absorbed water and is easily released, while bound water is linked to molecules [26]. In order to establish the amount of water present in aloe vera, the moisture percentage in fresh weight was determined (Table 1). The results show that the water quantity was similar to that described in the literature [1,16,27], between 98.5% and 99.6%. In fresh filets the moisture percentage was also similar to those described, [1,16]; that is, 98% and 98.8%, respectively.
| Characteristics | Freshgel | Pressed cake |
|---|---|---|
| Moisture(% fresh weight) | 97.84%±0.46 | 98.23%±0.66 |
| pH | 5.24 ±0.12 | 5.28 ±0.06 |
| Acidity (%malicaciddry weight) | 0.07%±0.01 | 0.08%±0.02 |
| Color | Paleyellow | - |
The figures are the mean of three independent measurements with ± standard deviation.
Table 1: Physical and chemical characteristics of fresh gel and pressed cake.
The pH in gel samples is slightly acidic, ranging from 5.0 to 6.5. Other results reported are different from ours, with a pH ranging from 3.5 to 4.7 [16,27]. The results indicate that Aloe vera plants under our conditions are able to buffer acidic pH. The pH range found in our results is that established by The International Aloe Science Council [21]. Other pH values reported in the literature were near 4.27 and 4.57. As a CAM plant, leaf pH will vary from day to night, since synthesis and accumulation of malic acid occurs at night [28].
Organic acids: In this study, organic acids were quantified by titration. Some organic acids found in plants are citric, tartaric and malic acids. Since Aloe vera is a CAM plant, most organic acid detected is malic acid due to C4-CO2 assimilation [29,30]. Acidity was similar in gel and in pressed cake (pH 5.24 in gel and 5.28 in cake). The relative malic acid content detected was 0.07% in gel and 0.08 % in cake. The IASC reported values of between 08% and 0.34%. Maintaining internal acidic pH in aloe vera is important, since higher pH make hydrocolloids (gel) susceptible to microbial contamination.
Color: The color of the fresh gel is a transparent pale yellow, with chromaticity coordinates of x=0.333 ± 0.000 and y=0.338 ± 0.000, (Table 1). Lyophilized gel and cake color analyses are important for commercialization, along with vitamin contents and nutritional values.
Antioxidant properties
The antioxidant capacity of lyophilized gel (Table 2), as determined by the DPPH radical discoloration method was good in comparison with other reported results [16]. Our samples indicated greater antioxidant capacity in fresh gel than in lyophilized gel. The difference between these two types of gels may be explained by higher solubility in the fresh gel in (80%) MeOH water alcohol while lyophilized gel could not be solubilized in this solvent, and therefore, could not act as an antioxidant.
| Gel type | Concentration (μg/mL) | Discoloration(%Inhibition) | Concentration(μg/mL) | Discoloration (% Inhibition) |
|---|---|---|---|---|
| Fresh | 160 | 11.61 ±0.53 | 680 | 46.67 ±0.35 |
| Lyophilized | 162 | 6.45 ±0.28 | 678 | 36.69 ±1.60 |
Values shown are the mean of three independent measurements ± standard deviation.
Table 2: Antioxidant capacity of fresh and lyophilized gel determined by the discoloration of the DPPH radical.
It should be noted that under moderate water restriction the superoxide dismutase activity of aloe vera increases, thereby reducing oxidative stress. The ascorbate peroxidase activity of aloe vera also increases under water and temperature stress [31]. The antioxidant capacity of fresh and lyophilized gels was determined at two gel concentrations (Table 2).
Proximal analysis
Proximal analysis or “Weend’s model” was used to determine the nutritional content of the gel and cake and is shown in Table 3. The moisture content of the gel (22.83%) was greater than the moisture content of the cake (6.87%). The gel consists mainly of polysaccharides that perform the physiological role of retaining water for the plant, as Aloe vera is adapted to aridity. The cake contains compressed leaf tissue without gel [16] reported 2.65% moisture in the gel, a very low value probably due to the storage conditions of the lyophilized gel. For example, maintaining the dried gel in flasks containing silica gel in paper envelopes to trap moisture will lower the amount of water in the gel. In pressed cake, the moisture value was greater than that reported by [16], who obtained a moisture value of 4.25%, while in this study the moisture value was 6.9%.
| Parameters | Freshgel ( %in fresh weight) | Cake (%indryweight |
|---|---|---|
| Moisture | 22.83 ±2.24 | 6.87 ±0.88 |
| Proteins | 6.11 ±3.47 | 9.43 ±0.64 |
| Ether extract | 2.07 ±0.61 | 1.61 ±0.50 |
| Fiber | 1.38 ±1.13 | 12.31 ±2.18 |
| Ash | 17.20 ±0.99 | 13.15 ±0.78 |
| Non-nitrogenous extract | 50.41 ±1.68 | 56.63 ±0.99 |
Analyses were performed for three separate determinations. Values shown are mean ± standard deviation.
Table 3: Proximal analysis of lyophilized gel and dehydrated cake.
The fiber in the cake was 9.4 times that of the gel, even though the gel consists of pectin and hemicellulose polysaccharides. However, the pressed cake contains other polysaccharides and polymers besides the pectins and hemicelluloses present in the gel, such as cellulose, other hemicelluloses and lignin, as the cake contains more cell walls than the gel. The nature of these polysaccharides remains to be determined. Since Aloe vera is a monocot, the hemicellulose of the cell wall could be an arabinoxylan polysaccharide [4,32,33]. However, it should be noted that the cake could be used as a food thickener or dietary fiber able to be included in food. Other authors [1] have described the amount of crude fiber in aloe vera released on treatment with acids and alkalis under standardized conditions, which underestimate fiber amounts, as alkalis and acids dissolve some cell wall polysaccharides such as hemicelluloses, cellulose, pectins and lignin polyphenols. Raw fiber has little physiological importance in human nutrition [34] due to the low amount of associated protein. However, it has other beneficial effects in the digestive system; it helps in diabetes, in lowering blood cholesterol levels and in preventing colo-rectal cancer.
Protein content (Table 3) was determined using Bradford’s colorimetric method [17]. Both the gel and cake contain protein; the cakes protein content was 1.54 times greater than that of the gel. Our results are similar to others reported using Kjeldahl?s method [1,16]. However, using Bradford?s method the amount of protein in the cake was twice that of the gel: 9.43% versus 4.27% as reported in the literature [16]. In any case, the protein content is low both in the gel and the cake compared to the polysaccharides present in the gel and cake [34].
Mass loss after incinerating organic residue left after sulfuric acid and sodium hydroxide treatments indicates that the cake has high fiber content (12.31%) compared with that of the gel (1.38%), because the cake is composed of cell walls of parenchyma tissue while the gel consists mainly of water-retaining soluble polysaccharides.
Dietary fiber
Determination of dietary fiber (Table 4) using the enzymegravimetric method is based on digesting proteins and carbohydrates with enzymes. The remaining product is dietary fiber (DF), after subtracting the amounts of ash. Although the total proteins in aloe are relatively few, their biological activities are meaningful as shown by their many clinical applications, even though their chemical structures are unknown [4]. One protein that has been described is carboxypeptidase, a 28 KDa or 56 KDa glycoprotein. Carboxypapetidases seem to be associated with the development of inflammation [4]. Under moderate water restriction, aloe vera also increases sugars and polysaccharides such as acemannans and fructans [35]. The molecular structures of both types of polysaccharides are associated with many medical, cosmetic and nutritional properties [36].
| Sample | SolubleFiber (%dryweight) | InsolubleFiber (%dryweight) | Total Fiber (%dryweight) |
|---|---|---|---|
| Dehydrated Cake | 17.54 ±2.11 | 53.92 ±7.31 | 71.46 ±4.71 |
| Lyophilized Cake | 16.58 ±1.81 | 48.22 ±2.64 | 64.80 ±2.23 |
The values are the mean of three independent determinations ± standard deviation
Table 4: Determination of fiber in the lyophilized and dehydrated cake.
Here, we determined the insoluble and soluble fibers obtained after alcohol precipitation [34]. One such soluble fiber molecule was acemannan.
DF is essential for normal intestine function. It consists of polysaccharides undigested by the small intestine that enter the colon, where they are fermented by the colon bacteria. This fermentation produces short chain fatty acids, increasing the population of beneficial bacteria such as bifid and lactobacilli bacteria and decreasing the numbers of dangerous coliform bacteria (prebiotic effect). Insoluble fiber moves rapidly in the gastrointestinal tract, increasing fecal material, accelerating intestinal transit and delaying glucose absorption. In contrast, soluble fiber forms a gel binding water, delaying gastric emptying and intestinal transit [37,38].
The results reported in Table 4 show that soluble and insoluble fiber is higher in dehydrated cake than in lyophilized cake, which has 7% less soluble fiber [16] reported 20% more total fiber (92.55% ± 4.82). However, our results indicate that the cake has a large amount of fiber regardless of the methodology used to dehydrate it. These results indicate that the cake may be used in the food industry as a valuable prebiotic.
Functional hydrocolloid properties are directly related with polysaccharide composition and structure, and are modified by molecular size, porosity, ionic form, pH, temperature, water quantity, etc. [35]. Table 5 shows that lyophilized gel has a high capacity for water retention, therefore increasing gel volume.
| Sample | Water retention capacity(WRC) (gwater/gdry weight) |
Swelling (SW) (mLwater/gdry weight) | Fat adsorption capacity (FAC) (g oil/g dry weight) |
|---|---|---|---|
| Lyophilized gel | 25.46 ±6.15 | 33.67 ±4.76 | 1.83 ±0.003 |
| Dehydratedcake | 20. 02 ±0.34 | 9.57 ±0.12 | 2.13 ±0.054 |
| Cortex | 18.00 ±0.60 | 13.42 ±0.13 | 1.50 ±0.057 |
Values are the mean of three independent determinations ± standard deviation.
Table 5: Functional properties of lyophilized gel, dehydrated cake and cortex.
Lyophilized aloe vera gel has a high WRC (25.46 ± 6.15 g water/g of lyophilized gel). However, Femenia [1] reported a WRC of 36 g of water/g lyophilized gel, indicating a retention 1/3 greater than ours. The difference probably occurs because samples were dried under different conditions, with a negative influence of high temperatures in cake and cortex dehydration [16,38].
Cake WRC was also high, at 20.2 g water per g dry weight, while cortex WRC was 18g water per g dry weight. These results confirm that Aloe vera is a CAM plant. Both gel and cake are polysaccharide-rich; acemannan is the gel?s main polysaccharide component. Acemannan is an acetylated galactoglucomannan with a high capacity for binding water using hydrogen bonds. Similarly, the cake contains cell wall polysaccharides such as pectins, and probably also glycosylated proteins with water retention capacity [39].
Polysaccharides
Aloe vera contains several polysaccharides, in which glucose, fructose and mannose are the main monosaccharide components. These polysaccharides are acemannan, in which mannose and glucose are the main components, and cellulose composed of glucose.
Other important polysaccharides are fructans, consisting of glucose and fructose. Acemannan [1] and fructans are the main beneficial polysaccharides in aloe vera [35]. In aloe gel, beta-(1,4)-acetylpolymannose intercepted with glucose is also known as acemannan, which is an acetylated glucomannan. Sometimes, the acemannan also contains galactose branches linked to mannose with α-(1,6) glycosidic linkage [40,41]. The glucomannan contains 97% water and 0.7% solids. However, significant variations were seen in pulp polysaccharide species in these early studies. For example, several studies identified acemannan as the major polysaccharide in the pulp [42]. Acemannans are hemicelluloses [39,42], whereas other studies found a pectic substance to be the primary polysaccharide in the absence of mannan [42]. The reasons for these discrepancies are not understood, but are probably largely due to seasonal change and/or the different geographic conditions of Aloe vera crops. In this experiment (Table 6), the values for lyophilized gel (expressed as mannans) were 400.0 ± 17.68 and 4.01% ± 0.18 respectively.
| Sample | Mannans (mg/g dry weight) |
Mannans (% dry weight) |
|---|---|---|
| Lyophilized gel | 400.0 ± 17.68 | 4.01 ± 0.18 |
| Dehydratedcake | 612.5 ± 26.52 | 6.13 ± 0.26 |
Values are the mean of three independent determinations ± standard deviation.
Table 6: Determination of polysaccharides of the lyophilized gel and dehydrated cake.
The colorimetric method used for quantifying polysaccharides in this study is based on the interaction of the polysaccharide with Congo Red to form a permanent molecular complex proportional to the sample glucomannan. This method is sensitive to small quantities of the polysaccharide. The 1% Congo Red solution in water absorbs light at 488 nm, but when Congo Red is combined with glucomannan, its absorption is 540 nm. Figure 1 shows the amount of mannan in the gel and cake.
The amounts of mannan found in each sample are shown in Figure 1, where mannan is 40% of the dry gel weight and 61.3% of the dry cake weight. This percentage is higher than previously reported figures [16], which were 16.3% for lyophilized gel and 18.42 for lyophilized cake.
The mannan content was still higher than in another report from the literature [23]; where the authors found it to be only 25%. Therefore, plants grown in semi-arid conditions in Chile have a higher mannan content.
Aloins
Our results indicate that aloin is present in the lyophilized gel, cake and cortex in very small amounts of 0.0228 %, 0.0117% and 0.0525% respectively. The higher amount of aloin in the cortex is due to the presence of cells adjacent to vascular bundles that synthesize aloin [43]. Zambrano et al. [44] Reported that aloin fluctuates from 0% to 0.107% in pure gel. In our results, aloin was less than 11% of 0.107. In the lyophilized cake, aloin was about 50% of that found in the gel (approximately 11.4 mg per L of gel) (Table 7). The literature reports [27] in the cake, about 50% of the amount of around 0.0058 reported in this paper .Aloin is water soluble, and its gel contains more water. Therefore, aloin probably contaminates the gel during filet preparation, remaining soluble in the gel, as the vascular bundles present in the center of the leaf where the gel is produced do not contain aloinproducing cells. The presence of aloin was further corroborated by thin layer chromatography [45].
| Sample | Aloin(%) |
|---|---|
| Lyophilized gel | 0.0228 ±0.018 |
| Lyophilizedcake | 0.0117 ±0.005 |
| Cortex | 0.0525 ±0.006 |
Table 7: Aloin quantification.
Aloin was determined in three independent samples of lyophilized gel, lyophilized cake and cortex. The values shown are the mean of these determinations ± standard deviation.
Mean gel yield was 86.3% of the whole filet, while freeze-dried gel corresponds to 0.91% of the volume of the fresh gel. The pressed fresh residue (cake) of the filet after gel extraction corresponded to 15.1% of the whole filet.
Fresh gel had moisture of 97.8%, a pH of 5.24 and 0.07% acidity expressed as malic acid. Water content analysis of the freeze-dried gel and dehydrated cake gave moisture values of 2.3% in the gel and 6.9% in the cake. Ash content was 17.20% in the gel and13.15% in the cake. Total dietary fiber determined in the cake (71.5%) was greater than that of the freeze-dried gel (64.8%). Water retention capacity (WRC), swelling (SW) and fat adsorption capacity (FAC) were greater in the cake than in the gel.
In summary, this paper describes a preliminary chemical characterization of Aloe vera (Aloe barbadensis Miller) leaf skin and gel, which were analyzed by dissecting entire leaves into filets and skin. Today, components of this plant are widely used in medicinal applications such as healing wounds, in cosmetics and as nutraceuticals (prebiotics). However, the methods by which the plant is processed mean that the quality and quantity of the components is variable [40] and therefore, depending on the methodology used, final preparations may have more or less beneficial properties for human health. Since many of the medicinal, cosmetic and nutritional properties of the gel and leaf skins depend on the polysaccharides and oligosaccharides present as components of the leaf, future studies should determine the sugar components and structure of these oligosaccharides and polysaccharides.