Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2014) Volume 4, Issue 2
β-Galactosidase was extracted (with acetate buffer pH 5) from apricot seeds, partially purified by ammonium sulphate (30-70%) and dialyzed for 24 hr. The crude extract had a higher specific activity (9.93 U/mg proteins) which indicates a high enzyme yield. Purification of crude extract with ammonium sulphate (30-70%) increased the specific activity and purification fold to 29.07 U/mg proteins and 2.92, respectively which was improved by dialysis to 32.68 U/mg proteins and 3.29, respectively. The extracted enzyme exhibits an optimum temperature at 70°C and 5 pH optima. The enzyme activity was almost stable between pH 5.0 and 6.5, where more than 90% of its activity was remained with incubation at the previous pH for 30 min. Further more; it was thermostable when incubated for 30 min. at temperature ranges of 55-70°C with a complete loss of activity at 80°C. The activity of β-galactosidase was enhanced by different concentration of Ca+2. There were no significant (P>0.05) changes in flavor, body texture, and overall acceptability between free lactose white cheese treated by apricot seeds β –galactosidase (9865 U/ 500 ml milk) and untreated cheese.
Milk is an extremely valuable and economic food containing high quality protein, calcium and phosphates and it also contains lactose [1]. Lactose is hydrolyzed by the lactase enzyme in the small intestine. Limited digestion of lactose can lead to unpleasant gastrointestinal symptoms of varying severity, termed lactose intolerance [2]. Lactose malabsorption / intolerance is common among approximately 70% of the world’s adult population which is caused by the intestinal insufficiency of the enzyme β-galactosidase/lactase [3,4]. Lactose intolerance is a clinical syndrome of 1 or more of the following: abdominal pain, diarrhea, nausea, flatulence, and/or bloating after the ingestion of lactose or lactose-containing food substances [5]. Individuals with these symptoms often begin to avoid milk and dairy products thus leads to a lower calcium intake [6], which is associated with reduced bone mineral density and an increased risk of developing osteoporosis [7]. Therefore individuals with lactose intolerance can consume free milk and dairy lactose products. Enzymatic hydrolysis of lactose by β-galactosidase is one of the most popular technologies to produce lactose reduced/free milk and related dairy products for consumption by lactose intolerant people [8-13]. Additionally, Lactosehydrolysed milk used for the preparation of yoghurt and cheese manufactory accelerate the acidification process that reduces the set time of yoghurt and develop the structure and flavor in cheese [14]. β-Galactosidases are found in microorganisms (bacteria, fungi, yeasts), plants especially in almonds, peaches, apricots, apples and animal organs [15-17]. The β-galactosidases extraction and purification from many microorganisms for the preparation of de-lactosed milk shown inhibition to Ca+2, an essential milk component occurring at the concentration of 30 mM, indicating that lactose hydrolysis with these preparations are not effective [18]. Therefore, this study was aimed at extraction, purification and characterization of β-galactosidase from apricot seeds and to explore the possibility of preparation of free lactose milk for preparing free lactose cheese suitable for lactose intolerance population.
Materials
Apricot seeds were obtained from Kaha Company for food industries kaluobia government, Egypt. Fresh buffalo’s milk was obtained from Faculty of Agriculture, Minufiya University. Onitrophenyl- β-D-galacto pyranoside (ONPG) and tri chloro acetic acid (TCA) was obtained from Sigma Chemical Co., St. Louis, MO, USA.
Kits for determine glucose was purchased from (Biosub, glu, Biocon, Germany). Bovine serum albumin and Folin-ciocalteau was obtained from Biochemical, BDH Chemicals Ltd Poole, England.
Methods
Enzyme extraction and partial purification: The grounded fresh apricot seeds (100 g) were homogenized for 60 min at 4°C with 500 ml of 0.02 M acetate buffer (pH 5). The homogenate was filtered and centrifuged (Jouan, MR, 18-12, France) at 5000 g for 15 min and supernatant referred to as crude extract. For preliminary assay; 50 ml of crude enzyme extract were used to determine the appropriate ammonium sulfate concentration for partial purification. The ammonium sulphate concentration (30-70%) which gave the highest purification fold and specific activity was used to purify the remaining initial extract through tow steps: first, the solution was brought to 30% concentration then centrifuged at 5000 g for 10 min and the precipitate discarded. Secondly, the enzyme solution was brought to 70% saturation and the enzyme precipitate retained and diluted to 100 ml 0.02 M acetate buffer (pH 5) then dialyzed against the same buffer for 24 hr at 4°C with changing the dialysis buffer twice.
Enzyme activity assay: The activity of β-galactosidase was assayed as described by Li et al., (1975) [19] using ONPG as a substrate. The reaction mixture consisted of 0.2 ml of ONPG, 0.7 ml of 0.02 M acetate buffer (pH 5) and 0.1 ml of β-galactosidase extract. The reaction mixture was incubated at optimum temperature for 30 min. The reaction was terminated by the addition of 0.5 ml of 1M sodium carbonate. The resulted O-nitrophenol was estimated spectro-photometrically (UNICO 2802 C/PCS Series spectrophotometer, USA) at 410 nm. One unite of β-galactosidase activity is defined as the amount of enzyme required to release 1 μmol of o-nitrophenol (ONP) per min under the condition described above.
Protein assay: Protein content was determined by the methods described by Lowry et al., [20] with bovine serum albumin as standard.
Specific activity of enzyme, purification fold and total activity yield were calculated as following:
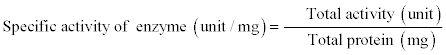
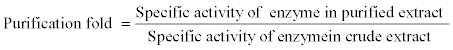
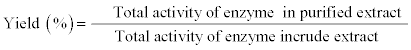
Enzyme kinetic methods
pH and temperature optima: The optimal pH of β-galactosidase activity was determined in the assay mixture with different pH ranging from 4-9 using acetate buffer (4.0-5.5), phosphate buffer (6-8) and boric acid borax buffer (8.5-9.0). The optimal temperature of enzyme activity was determined by incubating the assay mixture at pH 5 for 30 min at a temperature ranging from 40 to 80°C. The residual enzyme activity was determined as previously described in 4.2.2.
Thermo stability and pH stability: Enzyme extract was preincubated at temperatures in the range of 40-80°C for 30 min at optimum pH of β-galactosidase extract for thermo stability evaluation. For pH stability, enzyme extracts were pre-incubating at pH values in the range of 4.0-9.0 for 30 min at optimum temperature of β-galactosidase extract. The residual enzyme activity was determined as previously described.
Effect of Ca ions: Stock solutions of CaCl2 (prepared in 0.02 M acetate buffer pH 5) were added separately to the reaction mixture to a final concentration of 0.001, 0.002, 0.003, 0.0004 and 0.005 mol/Liter. The residual enzyme activity was assayed and expressed as a percentage of the activity determined in acetate buffer alone (control).
Preparing of free lactose soft (white) cheese: Fresh buffalo’s milk was obtained from the herd of the faculty of agriculture, Menoufya University, Shibin El-kom, Egypt. Fat content was standardized to 6%. fifty ml (9865 units) of partially purified β-galactosidase apricot seed extract was added to five hundred ml of fresh buffalo milk and stirred gently with glass rood at ambient tempearature (25 ± 2°C) for 5 min then milk was heated at 68-70°C for 30 min and cooled immediately to 38-40°C. Calcium chloride were added at the rate of 0.02% before renneting [21]. Samples were taken from fresh and pasteurized as well as the produced cheese and the liberated glucose was determined using the glucose oxidase reagent kit (Biosub, glu, Biocon, Germany).
Sensory evaluation of white cheese: Salted (4% w/w) white cheese was organoliptically evaluated for flavor, taste, appearance and texture by 15 panelists (staff member of food science and technology department).
Statistical analysis: The results recorded as the mean ± SD of eight replicates. The experimental data were subjected to an analysis of variance (ANOVA) for a completely randomized design using a statistical analysis system [22]. Duncan’s (1995) multiple range tests were used to determine the differences among means at the level of 5%.
Partial purification of β-galctosidase
The purification steps, protein concentration, specific activity and yield, of apricot seeds β-galactosidase are showed in Table 1. The crude extract had a high specific activity (9.93 U/mg protein) which indicate a high enzyme yield higher than that reported for other sources, 0.056 U/mg protein for mung bean [23] and 3.1 U/mg protein for kidney bean [24]. Purification of crude extract with ammonium sulphate (30-70%) recovered 87.34% of the extracted enzyme and improved the specific activity and purification fold to 29.07 U/mg proteins and 2.92, respectively. These results agree with those reported by Shaheen et al., [25] who purified β-galactosidase from soybean using ammonium sulfate precipitation with a 5.1-fold increase in specific activity and 38.3% recovery. Furthermore, the collected precipitate from the ammonium sulphate precipitation step was dissolved in acetate buffer pH 5 and dialyzed for 24 hr. against the same buffer. The dialysis step increased the specific activity to 32.68 U/mg proteins and the purification fold to 3.29.
| Purification steps | Total volume | Total activity | Total protein | Specific activity U/mg protein | Recovery % | Purification fold |
|---|---|---|---|---|---|---|
| Crude extract | 500 | 98650 | 9936 | 9.93 | 100 | 1.00 |
| Ammonium sulphate (30-70) | 100 | 86200 | 2965 | 29.07 | 87.34 | 2.92 |
| Dialysis | 100 | 84850 | 2596 | 32.68 | 86.01 | 3.29 |
Table 1: Purification steps of apricot seeds β-galactosidase.
Optimum pH
The β-galactosidase activity was found to vary with pH values (Figure 1). The optimum pH of the enzyme activity was 5.0. The activity of the enzyme decreased gradually at pH values higher or lower than the optimum pH value, retaining more than 50% of its maximum activity at pH range of 4- 6.5. The increasing of the pH over 7 drastically decreased the enzyme activity. These results agree with the observation reported by Mc Gee and Murray [26] that the pH optimum of plant β-galactosidase lies in the acidic range while those derived from bacteria are in the neutral range. Gulzar and Amim [27] extracted three isoenzymes of β-galactosidase from apricots and they found that the optimum pH was found between 4.0 and 6.0. The pH optimum of β-galactosidase extracted from peach was 3.0 [28] while it was 5.5 for that extracted from almond [29].
Optimum temperature
The apricot β-galactosidase activity increased with increasing the temperature up to reaching maximum activity at 70°C (Figure 2). On further increase in temperature, the enzyme activity declined gradually and at 80°C, enzyme exhibited 11.56% of the maximum activity. On the other side, the enzyme retained more than 60% of its activity at temperatures ranged from 40-65°C. The loss of activity of the enzyme at higher temperatures could be attributed to its unfolding and subsequent loss of active site [17]. The optimum temperature of β-galactosidase was in the range 40-60°C [27,28,30].
pH stability
The pH stability study is an essential part of any enzyme characterization before it can be exploited commercially. The results shows that pre-incubation of β-galactosidase in the pH range 5.0 to 6.5 has no almost effect on the activity measured at its optimum pH (Figure 3). However pre incubation at 5.0 pH > 7.0 result in a decreaseof the activity of β-galactosidase. Similar results were reported by Pal et al. [29]. When the enzyme is pre-incubated at pH > 6.0 or pH < 5.0, full activity is not regained at pH 5.5. Thus, part of decline in activity above pH=6.0 and below pH=5.0 may be results from irreversible enzyme inactivation [29]. It is well–know that pH of milk is 6.14. The results showed that at pH 6.5 the β-galactosidase remain more than 90% of the maximum activity, of pH optimum of enzyme which is suitable for hydrolysis of lactose present in milk. The pH stability of β-galactosidase from fruits was at 3.0-10 range for peach [28], between pH 2.0 and 6.0 for persimmon [31]. The apricot β-galactosidase possess a wide range of pH stability [27].
Thermo stability
Figure 4 shows the effect of temperature on stability of β-galactosidase. The results revealed that the activity β-galactosidase remained constant when incubated at temperatures ranging from 55- 70°C for 30 min. The enzyme showed a sharpe decrease in activity after word. At 80°C there was complete loss of the enzyme activity. Similar results were reported by Sun et al., (1999) [1] who noted that the thermo stability of β-galactosidase isolated from gram chicken bean was at 50-70°C range. However these results are in close agreement with that reported for the β-galactosidase from other fruits [28,32].
Effect of different calcium concentration on the β-galactosidase activity
Activity of enzyme increased with increasing the concentration of calcium up to reaching at 0.005 which the activity Figure 5 shows the effect of different concentration of calcium on the β -galactosidase activity. The results indicated that the calcium stimulated the enzyme activity by 1.65, 3.55, 4.85 and 7.0% at concentration of 0.001, 0.002, 0.003 and 0.004 and respectively. However at concentration of 0.005 the activity of enzyme was 2.94%. These results are agreement with Pal et al., [29] who reported that the activity of β–galactosidase extracted from almond seeds increased by different concentration of Ca+2. Calcium is one of the important intrinsic components of milk and stimulation of enzyme activity by this divalent cation is good at practical point of view since it will facilitate the hydrolysis of lactose [29]. In contrast to apricot seed β-galactosidase, microbial β-galactosidases have been reported to be inhibited by Ca+2, thereby restricting their application.
Effect of apricot seeds β -galactosidase treatment on lactose content of white cheese
Table 2 illustrates the percentage of milk lactose hydrolysis as affected by partially purified apricot seeds B-galactosidase (9865 units). Generally, treating the milk with apricot B- galactosidase highly hydrolyzed the milk lactose thus hydrolysis increased with increasing the treatment time. The produced cheese exhibit the highest lactose hydrolysis (99.66%) which was too close to that of whey (99.81%) compared to 0.319 % and 6.68% for the untreated samples. The high optimum temperature (70°C) of the enzyme help to increase the lactose hydrolysis even during the milk pasteurization processes 91.37% compared to 0.322% for the pasteurized milk without enzyme treatment. Our results is too close to that reported by Hussein, 2004 [33] who concluded that the milk lactose could be hydrolyzed completely by incubation with E. Coli B-galactosidase for 3 hr, and higher than that reported for Sun et al., 1999 [1] who noted that only 75% of lactose could be hydrolyzed by incubation with chicken bean B-galactosidase for 4 hr.
| Fresh milk | Pasteurized milk | White cheese | Whey | |
|---|---|---|---|---|
| Control | 0.319a ± 0.3 | 0.322b ± 0.02 | 6.12b ± 0.38 | 6.68b ± 0.03 |
| Treated milk | 0.319a ± 0.32 | 91.37a± 3.06 | 99.66a ± 1.28 | 99.81a± 0.08 |
| LSD | 0.072 | 4.89 | 3.27 | 0.15 |
Means ± SD values in columns followed by different letters in the same characteristic are significantly different (P ≤ 0.05).
LSD: least significant difference.
Table 2: Effect of apricot seeds β - galactosidase treatment on lactose contents of produced cheese.
Sensory attributes of reduced lactose white cheese
No significant (P>0.05) differences were observed in flavor and body texture between white cheeses treated with β- galactosidase and the untreated samples (Table 3). On the other side, treating with β-galactosidase enzyme reduced (P ≤ 0.05) the acceptability of taste and appearance with keeping the overall acceptability without significant changes. These results support using of such enzyme for preparing the reduced lactose white cheese.
| Sensory characteristics | Treated cheese | Untreated cheese | LSD |
|---|---|---|---|
| Flavor (25) | 22.87a | 22.46a | 1.22 |
| Taste (25) | 20.75b | 23.29a | 1.83 |
| Body texture (25) | 23.11a | 21.05a | 2.05 |
| Appearance (25) | 22.32b | 23.78a | 1.23 |
| Overall acceptability | 89.05a | 90.58a | 2.10 |
Means values in the same rows followed by different letters are significantly different (P ≤ 0.05).
least significant difference.
Table 3: Sensory characteristics of white cheese prepared with β-galactosidase.
The presented result seems to be (as our knowledge) the first to determine the extraction, partial purification and characterization of apricot seeds β-galactosidase. The observed higher yield and specific activities as well as its thermal stability can be considered an important candidate for the production of a novel β-galactosidase allows us to achieve a high level of lactose hydrolysis. The enzyme optimum temperature as well as its pH stability is too suitable for using to hydrolyze milk lactose. Further purification and more detailed characterization of the enzyme from apricot seeds is currently in progress.
We gratefully appreciate the technical support of Dr. K. Kebary and staff member of dairy science department faculty of agriculture, Minoufiya University.