
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Case Report - (2022)Volume 12, Issue 3
Background: Granulosa Cell Tumors (GCTs) arising outside the ovary are extremely rare. Only 7 cases were reported previously. The clinical characteristics, surgery, therapy, prognosis, pathology and origin are unknown. This is the first case reported of extra ovarian GCT arising from ileum.
Case presentation: A 44-year-old nulliparous Chinese female presented with weight loss, anorexia and hiccups for 3 months. Surgeries revealed a 8 × 7 × 6 cm, solid mass arising from distal ileum, separating from the adnexa and uterus of normal appearance. Postoperative pathology confirmed the primary extra ovarian GCT of ileum. Only one month of disease-free after four cycles of postoperative chemotherapy was remained when metastatic tumor developed in liver.
Conclusion: GCTs can arise from tissues other than ovary, may be with poor prognosis, and origin from mesenchymal tissues of embryologic genital ridge.
Extraovarian GCT; Ileum; Poor prognosis; Postoperative pathology
GCT: Granulosa Cell Tumor; SCST: Sex Cord Stromal Tumor; EGR: Embryologic Genital Ridge; CA125: Carbohydrate Antigen 125; CA199: Carbohydrate Antigen 199; AFP: Alpha Fetoprotein; CEA: Carcinoembryonic Antigen; β-HCG: Beta-Human Chorionic Gonadotropin; PET-CT: Positron Emission Tomography- Computed Tomography; SUV: Standard Uptake Value
Granulosa cell tumors (GCTs), the most common type of sex cord stromal tumors (SCST), are considered to originate from the proximal precursors of ovarian sex cord and occur mainly in ovary [1]. However, the extremely rare cases of primary extraovarian GCT have been reported. It is well established that only 7 such extraovarian GCT cases have reported in English literatures, including pelvic sidewall, fallopian tube, broad ligament, uterosacral ligament, cerebellum, mesentery and retroperitoneal space [2-8]. As result, another theory of GCT origin that it is from mesenchymal tissues of Embryologic Genital Ridge (EGR) has been considered [9]. Nevertheless, in our case, the GCT arising from ileum with normal adnexa and uterine is reported first.
A 44-year-old nulliparous Chinese female without personal or family history of disease was admitted to the Hubei cancer hospital in October 2021, who presented with weight loss, anorexia and hiccups for 3 months. On physical examination, an approximate 10 cm, painless, mobile, suprapubic and right-pelvic mass was revealed. On inspection, occult blood was detected in stool, while preoperatively electronic gastroscopy and colonoscopy revealed no tumor lesion. The level of serum CA125 elevated to 187.6 umol/ ml. Serum sex hormone and other tumor markers including AFP, CA199, CEA, β-HCG and inhibin were within normal range. Preoperative PET-CT, as shown in Figure 1, demonstrated a 7.8 × 6.1 × 6.3 cm, solid and right-pelvic mass with SUVmax of 11.22, without clear boundary to adjacent intestine or right adnexa. Cervical cytology endocervical curettage was normal. The mass was diagnosed for ovarian tumor initially, followed by laparotomy.
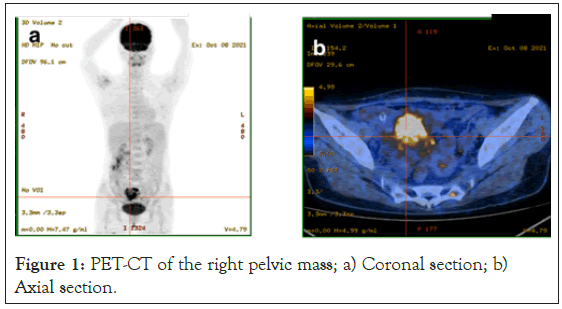
Figure 1: PET-CT of the right pelvic mass; a) Coronal section; b) Axial section.
The primary laparotomy revealed a 8 × 7 × 6 cm, solid mass arising from distal ileum, separated from the right adnexa and adherent to the adjacent mesentery and small intestine, resulting in intestinal stricture. The uterus, adnexa, omentum and peritoneum appeared grossly normal. 63 cm small intestine with mesentery and the mass was resected merely in toto, and primary ileal mesenchymal tumor with whole intestinal layer invasion was suggested by the intraoperative frozen section. Secondary laparotomy was performed 3 weeks later, following postoperative pathologic confirmation of ileal GCT. No visible lesion was explored in abdominopelvic cavity, except several 1-2 cm solid nodules in Douglas space. This surgery without tumor residue involved total hysterosalpingooophorectomy, omentectomy and pelvic floor peritonectomy. No evidence of tumor was identified in adnexa, uterus or omentum in microscopy, but only GCT metastasis was detected in Douglas space peritoneal nodules. Primary GCT of ileum was diagnosed by intraoperative exploration and postoperative pathology. Four cycles of postoperative intravenous-chemotherapy with paclitaxel and cisplatin was carried out. The patient remained only one month of disease free when metastatic tumor developed in liver.
Postoperative pathology revealed the primary GCT of ileum. Para-cancerous normal mucosa glands of ileum shown in Figure 2a, suggesting the location of GCT in ileum. Microfollicular containing eosinophilic material, gland-like arrangement with pyknotic nuclei and resembling Call–Exner bodies were noted in tumor tissue in Figure 2b and Figure 2c. The nuclei of the majority of tumor cells were formed of pale chromatin with nuclear grooves shown in Figure 2d. Sparse reticulin staining around perivascular area was shown in Figure 3a. Immunohistochemistry (IHC) results at CD56 (+), CD99 (+), Inhibin (+), Ki-67 (+, Li: 10%), Vimentin (+), CK7(-), EMA (-), Desmin (-), and the positive IHC were shown in Figure 3b-Figure 3f. Bilateral adnexa were entirely submitted for pathologic review using 3-5 mm sections. No GCT cell was identified.
Figure 2: Scanning microscopy for HE stain: a) para-cancerous normal mucosa glands of ileum indicated by arrows (✖100); b) resembling Call– Exner bodies (✖200); c) microfollicular containing eosinophilic material, gland-like arrangement with pyknotic nuclei (✖200); d) diffused GCT cells formed of pale chromatin with nuclear grooves (✖400)
Figure 3: scanning microscopy for reticulin stain and IHC positivity (✖400): a) Sparse reticulin around perivascular area (reticulin stain, ✖400); b) CD56; c) CD99; d) Inhibin; e) Ki-67; f) Vimentin.
The extraovarian GCT is extremely rare, through review, only 7 cases have reported in the literatures [3-9]. However, none of them was primary GCT of ileum, as shown in Table 1. In general, GCTs are usually accompanied by estrogenic preponderance, such as menstrual disorder, elevation of serum estrogen, endometrial hyperplasia and so on [10]. While, no estrogenic preponderance was observed in this case. It is suggested that the extraovarian GCT cells could not be provided with hormonal activity possibly.
| Case | Age (years) | Primary location | Source |
|---|---|---|---|
| 1 | 69 | Right fallopian tube | Barbosa LC, et al. [3] |
| 2 | 67 | Right pelvic sidewall | Robinson JB, et al. [4] |
| 3 | 71 | Cerebellum | Rauniyar S, et al. [5] |
| 4 | 20 | Uterosacral ligament | Pun JJ, et al. [6] |
| 5 | 27 | Broad ligament | Shone N, et al. [7] |
| 6 | 54 | Mesentery | Naniwadekar MR, et al. [8] |
| 7 | 69 | Retroperitoneal space | Vasu PP, et al [9] |
Table 1: The location of extraovarian GCTs cases reported previously.
Ovarian GCT is a malignant tumor with a tendency for late recurrence and an unpredictable response to adjuvant chemotherapy. Early recurrence is always unusual [1]. However, in this case, newly metastatic nodules were developed in peritoneum less than 3 weeks after the primary surgery, inferring the poorer prognosis compared with ovarian GCTs. As a result, four cycles of following postoperative chemotherapy was performed. Moreover, the early recurrence in liver after chemotherapy verified the poor prognosis of extraovarian GCTs.
The most possibilities exist regarding the pathogenesis of extraovarian GCT, including missed ovarian GCT and uterine stromal tumor. A missed diagnosis of GCT in the ovary is excluded, since intraoperative exploration and complete sectioning of bilateral adnexa revealed no evidence of tumor. Moreover, a thorough pathologic evaluation of uterus eliminated other undistinguishable diagnoses, especially uterine tumor resembling ovarian sex-cord tumors [11]. This pathologic diagnosis of ileal GCT was based on diffuse sex cord cells, Call-Exner body-like microfollicular, the uniform epithelioid cells with nuclear grooves, and para-cancerous normal mucosa glands of ileum. Reticulin stain and IHC-positivity of CD56, CD99, Inhibin, Ki-67 and Vimentin were supportive for GCT diagnosis [12].
The origin of primary extraovarian GCTs is unclear so far. The origin may be from mesenchymal tissues of EGR or the ectopic ovarian tissues. Assume the latter is true, the ovarian tissues would be discovered by pathology unless all the ectopic tissues had been replaced by tumor. While, no evidence of ectopic tissues was revealed in our case. As a result, we prefer to have faith in the former theory. Although this one case cannot prove it, it does provide evidence that certain GCTs could arise outside the ovary.
Primary GCTs could rarely arise outside the ovary, without estrogenic preponderance. The extraovarian GCTs could deserve aggressive treatment, because of the poor prognosis. It’s origin maybe from mesenchymal tissues of EGR.
Consent was obtained from the patients and from her legal representatives for participate in this study.
We obtained the patient’s and legal representative’s consent for publication of this case report.
All the data are available in the medical record.
All the authors declare that they have no competing interests.
Zhang HF researched the manuscript. Zeng R wrote the manuscript. Huang W and Hu P contributed to be involved in the clinical practice of the patient. Guo F contributed to be involved in the pathological diagnosis of the patient. All the authors reviewed the manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rong Z, Wei H, Peng H, Fang G, Huifeng Z (2022) Extraovarian Granulosa Cell Tumor with Call-Exner Body-like Microfollicular Arising From Ileum: A Case Report and Review of Literature. J Clin Trials. 12:499.
Received: 18-May-2022, Manuscript No. JCTR-22-17557; Editor assigned: 20-May-2022, Pre QC No. JCTR-22-17557 (PQ); Reviewed: 03-Jun-2022, QC No. JCTR-22-17557; Revised: 10-Jun-2022, Manuscript No. JCTR-22-17557 (R); Published: 17-Jun-2022 , DOI: 10.35248/2167-0870.22.12.499
Copyright: © 2022 Rong Z, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.