Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
+44 1300 500008
ISSN: 1920-4159
+44 1300 500008
Research Article - (2024)Volume 16, Issue 5
In this study, the use of two modified chitosan derivatives (CS-ODEX and CS-OCNC) for the fabrication of mucoadhesive tablets for the oral delivery of Omeprazole (OME), is described. These biopolymers were synthesized by grafting dextran and nanocellulose onto chitosan, aiming at enhancing the hydrophilicity and potentially improve the mucoadhesive properties of the OME tablets. The prepared tablets were characterized using various techniques, including microscopy, ATR-FTIR, XRD, swelling profile, and contact angle measurements. The results confirmed the successful synthesis of the chitosan derivatives and the formation of tablets with smooth surfaces. ATR-FTIR analysis did not indicate any strong drug-polymer interactions, while XRD suggested the amorphous dispersion of OME within the tablets. Swelling studies revealed a dependence on the type of derivative used, with CS-ODEX exhibiting the highest swelling capacity. The in vitro cytotoxicity of the chitosan derivatives was evaluated using human periodontal ligament cells, demonstrating their biocompatibility. In vitro drug release experiments were conducted, and a release mechanism is proposed. The study highlights the potential of utilizing modified chitosan derivatives with an increased number of hydroxyl groups for developing mucoadhesive tablets for the oral delivery of OME. The findings suggest that the present approach is promising for enhancing drug bioavailability and potentially improving the therapeutic efficacy of OME.
Omeprazole; Chitosan; Modified release matrix tablets; Mucoadhesion; Sustained release
Omeprazole (OME), 5-methoxy-2-[[(4-methoxy-3,5- dimethylpyridin-2-yl)methanesulfinyl]-1H-benzimidazole], is a proton pump inhibitor. It is administered for the treatment of dyspepsia, gastro-duodenal ulcers, symptomatic gastro-esophageal reflux, and the Zollinger-Ellison syndrome. The oral drug delivery of pharmaceutical dosage forms intended for managing and treating several conditions of the gastrointestinal tract constitutes a reliable administration route with well-established advantages [1,2]. In general, the oral dosage forms are convenient to swallow, cost-effective and appropriate for a plethora of active substances. They are also easy to transport and store, always ready to administer and above all, they can actively facilitate patient compliance [3].
However, the oral administration of OME faces some physiological constraints imposed by the physiology of the gastrointestinal tract (e.g., the pH, commensal flora, gastrointestinal transit time, enzymatic activity) [4]. To overcome some of these inherent physiological variabilities, the development of mucoadhesive drug delivery systems, aiming at the modified-release dosages forms, has become very popular. Such systems exhibit numerous benefits like, amongst others avoiding first-pass metabolism and dose-related side effects, enhancing permeation and preventing enzymatic degradation. During the last two decades, various mucoadhesive polymers have been investigated for this type of applications [5-7]. In general, mucoadhesion is defined as the interaction between a mucoadhesive polymer and the mucosal layer, and these theories describe various steps of the interaction between two substrates.
Bioadhesion seems particularly appealing for preparing controlled drug delivery systems aiming at enhancing the systemic or local administration of drugs via the intraoral route. A tablet possessing mucosal adhesion properties can extend the drug's adherence duration at the body's absorption site, thereby regulating drug release, enhancing drug absorption and augmenting the therapeutic effectiveness of the drug [8]. The adhesive mucosal dosage forms, which have been suggested for oral delivery include adhesive tablets, the strong adhesion of which to the mucosa is being achieved by the use of mucoadhesive polymers. Various synthetic polymers including cellulose derivatives, plant gums and polyacrylic acid have been described to exhibit adhesive behavior [9]. Furthermore, natural polymers as drug carriers have received significant attention in the pharmaceutical field from the viewpoint of safety and excellent biocompatibility [10].
Alginate is a naturally occurring block copolymer consisting of two monosaccharide moieties, mannuronic acid and guluronic acid, obtained from the marine brown algae. Sodium alginate is a biodegradable and biocompatible polysaccharide. It has been used in various dosage forms with different release mechanisms, such as mucoadhesive systems due to its capability to induce significant bioadhesion with the mucosal membrane [11]. Moreover, it is extensively used in the pharmaceutical industry as an excipient for tablet formulations since it is an excellent tablet binder [12].
Chitosan (CS) belongs to the first generation of mucoadhesive polymers. CS is a biodegradable polysaccharide with diverse and discrete biological properties, such as enhanced biocompatibility, biodegradability, nontoxicity, increased immunity, antimicrobial activity, thus exhibiting enormous potential in the biomedical sector [13]. The rising interest in using CS, in tablets’ formulations, is derived from its ability to increase penetration through the mucosal tissues by opening tight junctions and also its bioadhesive behavior [13]. Chitosan’s mucoadhesive character has already been extensively studied and has been shown that the positive charge of its amino groups allows the ionic binding with the anionic components of the mucosal surface, benefiting a greater interaction and adhesion [7,13]. Interestingly enough, there is further evidence that chitosan’s mucoadhesive behavior derives not only from its cationic character, which allows electrostatic binding to negatively charged mucins, but also by hydrogen and hydrophobic bonding. Thus, re-defining the structure of chitosan backbone by integrating functional groups through chemical modification is an attractive route to control the aforementioned characteristics and tune the mucosal oral delivery.
CS can be easily modified to enhance its low water solubility, due to the presence of the amino and hydroxyl groups. This modification is of significant interest since it would not alter the fundamental framework of CS and at the same time, retain the original physicochemical and biochemical properties and ultimately enhance its properties [14]. Sogias et al., reported the fabrication of mucoadhesive tablets for the oral delivery of ibuprofen using chitosan and its half-acetylated derivative [15]. It was found that the swelling capability and the drug release were higher with the half-acetylated chitosan tablets than from the tablets containing only the virgin polymer. Hauptstein et al., investigated the effect of thiolation on adhesion of chitosan’s compressed discs to porcine intestinal mucosa [16]. The group synthesized PEG-bearing thiolated CS via conjugation of the thiol-bearing polyoxyethylene ligand [O-(3-carboxylpropyl)-O′-[2-(3-mercaptopropionylamino) ethyl]polyethyleneglycol] to the amino moieties of CS. In addition to its solubility in basic medium, PEG-bearing thiolated chitosan presented enhanced mucoadhesive properties compared to those of the unmodified CS. Similarly, our group has previously reported the advantages of utilizing thiolated chitosan in sustained-release drug delivery applications [17]. Martin et al., synthesized palmitoyl glycol CS with various degrees of palmitoylation [18]. It was found that by increasing the hydrophobicity (dependent on the degree of palmitoylation), the erosion and hydration of the gels were reduced. Nevertheless, bioadhesion could be improved by increasing hydrophobicity. The most hydrophobic palmitoyl glycol CS gel (20.31% ± 2.22 mol % palmitoylation) led to the slowest sustained release of the model hydrophilic drug.
Dextran (DEX), another natural polysaccharide composed of D-glucose, is biocompatible and biodegradable. Some of the biofunctions of DEX include wound healing, haemostasis and inhibition of bacterial growth [19].
Cellulose is the most abundant natural biopolymer, with a primary function as a bio-based reinforcing nanofiller. Cellulose Nanocrystals (CNC) have attracted considerable interest in the past twenty years due to their numerous advantages, which have been widely used to enhance the properties of various host matrices in the preparation of nanocomposites [20]. Moreover, both molecules contain an abundance of hydroxyl groups that could significantly impact chitosan’s performance in terms of hydrophilicity and hydrogen bonding formation capacity, and thus possibly mucoadhesion, upon their grafting.
Although the combination of alginate and chitosan in oral mucoadhesive drug delivery has been reported, the chemical modification of CS with dextran and CNC, as well as their use in producing a modified-release tablet formulation for the potential treatment of gastrointestinal diseases is investigated herein for the first time, using OME as the active substance [21-27].
Material
Chitosan of high molecular weight (310,000-375,000 Da) and a degree of a deacetylation >75% was supplied from Sigma Aldrich Co (St. Louis). Acetic Acid (C2H4O2) of ≥ 99, 7% purity was purchased from Sigma Aldrich Co (St. Louis). Dextran (C6H16O5) with molecular weight ca 150.000 Da, was purchased from Thermo Fischer Scientific (Walltham) (CAS Number: 9004-54-0). Nanocellulose (C6H10O5) was obtained from Sigma Aldrich Co (St. Louis) (CAS Number: 9004-32-4). Sodium Hypochlorite (NaClO) CAS Number: 7681-52-9) was purchased from Sigma Aldrich Co (St. Louis). Tempo free radical (C9H18NO) (CAS Number: 2564-83-2) of 98% purity, was obtained from Thermo Fischer Scientific (Walltham). Sodium alginate was purchased from Alfa Aesar GmbH & Co KG (Karlsruhe), lactose monohydrate from Merck (Darmstadt) and magnesium stearate from Riedel-De Haen (Hannover). All reagents were of analytical grade.
Synthesis of chitosan derivatives
The two-step synthetic process (i.e., the oxidation of dextran/ nanocellulose and the modification of chitosan) of the modified chitosan derivatives is described below.
Oxidation of dextran: For the preparation of Oxidized Dextran (ODEX), 5 g of dextran were reacted with sodium periodate (1:1 molar ratio) in 400 mL of double-deionized water, for 20 h, at ambient temperature, and in the dark, to oxidize the Hydroxyl groups (-OH) to the respective Aldehydes (-CHO). Then, the requisite amount of ethylene glycol was added to the mixture, to terminate the oxidation reaction. The mixture was then dialyzed in a dialysis membrane for 3 days and then frozen and lyophilized using a freeze-drier system (Scanvac, Coolsafe 110-4 Pro, Labogen Scandinavia) for 24 h, at -108°C, to obtain a sponge-like dried material.
Oxidation of Cellulose Nanocrystals (CNC): The oxidation of nanocellulose was performed in the presence of the nitroxyl radical, 2,2,6,6-tetramethylpiperidinyloxy (TEMPO). Thus, 0.5 g of CNC was sonicated for 15 min in double-deionized water (50 mL). The requisite amounts of TEMPO and NaBr were dissolved in 50 mL of double-deionized water and then added dropwise to the CNC suspension. A small amount of a NaClO solution (12% wt) was slowly added to the resulted mixture to initiate the oxidation reaction. The pH of the TEMPO-CNC mixture was adjusted to 10 by adding 0.5 M NaOH, and the suspension was mechanically stirred for 3 h at ambient temperature. The oxidation reaction was terminated by the addition of ethanol (ca 1 mL) and the pH was adjusted to 7, using 0.5 M HCl. The desired compound, the oxidized CNC(O-CNC), was obtained via lyophilization.
Chemical modification of CS with O-DEX and O-CNC: The modified derivatives with oxidized Dextran (CS-ODEX) and oxidized nanocellulose (CS-OCNC) were prepared by the following procedures, following previous reports [28]. Specifically, the appropriate amount of chitosan was dissolved in an aqueous acetic acid solution (1% v/v), to form a 2% w/v chitosan solution, which was stirred overnight, at ambient temperature. The O-DEX or O-CNC, respectively, was then added, in the same amount (2% w/v) with the neat CS neat, and the two ingredients were left to spontaneously react under magnetic stirring for 24 h. The obtained hydrogels were frozen and then freeze-dried, until sponge-like materials were obtained.
Fabrication of CS-based tablet formulations
OME-loaded tablets were prepared via the compression of synthesized CS derivatives with the API and the respective excipients from Table 1. Initially, CS and its derivatives were placed in a porcelain mortar, where they were grounded into powder, using a pestle. The powder mixture was precisely weighed (200 mg), put into an evacuable pellet die (diameter: 13 mm), and compressed immediately with a Manual Hydraulic Press (Specac Ltd.,) at 10 tons for 4 minutes (Table 1).
| Sample | Weight (mg) | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| Omeprazole | 20 | 20 | 20 |
| Chitosan | 13 | - | - |
| CS-ODEX | - | 13 | - |
| CS-OCNC | - | - | 13 |
| Sodium alginate (Medium viscosity) | 75 | 75 | 75 |
| Lactose monohydrate | 90 | 90 | 90 |
| Magnesium stearate | 2 | 2 | 2 |
| Total (mg) | 200 | 200 | 200 |
Table 1: Composition of the prepared CS derivatives tabled with OME.
The prepared CS-OME tablets were observed under a ZEISS SteREO Discovery V20 microscope, and pictures were taken with a Jenoptik ProgRes GRYPHAX Altair camera equipped with a Gryphax image capturing software (Figure 1).
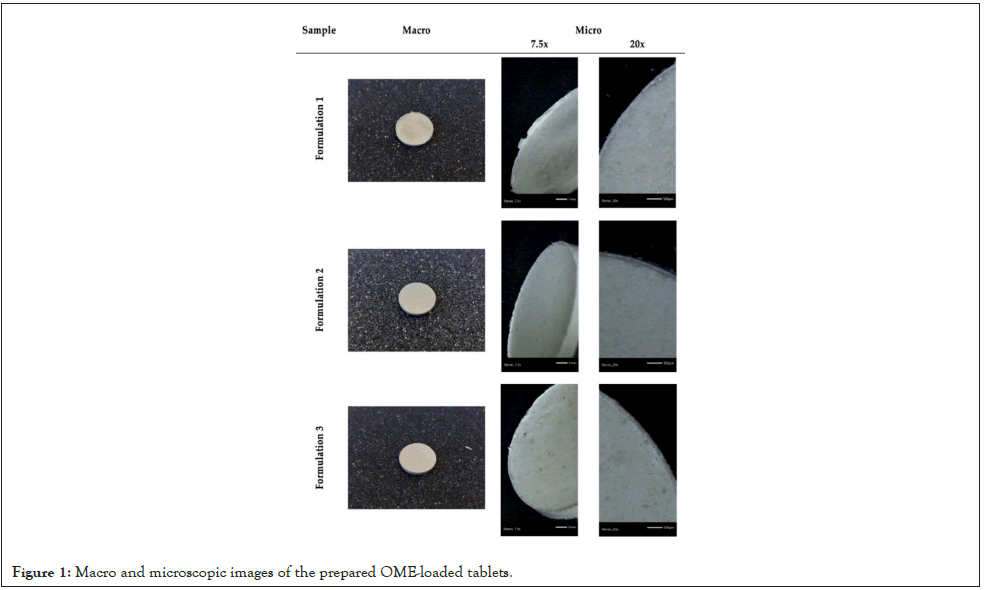
Figure 1: Macro and microscopic images of the prepared OME-loaded tablets.
Characterization of CS omeprazole tablets
Attenuated Total Reflectance-Infrared Spectroscopy (ATRFTIR): The ATR spectra of the samples were recorded using an IRTracer-100 (Shimadzu) equipped with a QATR™ 10 Single- Reflection ATR Accessory with a Diamond Crystal. The spectra were taken in the range of 450 to 4000 cm-1 at a resolution of 2 cm-1 (a total of 16 co-added scans), while the baseline was corrected and converted into absorbance mode.
X-Ray diffractometry (XRD): The crystallinity of the samples was studied by wide-angle X-ray diffraction, using the Rigaku Miniflex II diffractometer with CuKα radiation (k=0.154 nm). In order to obtain the diffractograms, the appropriate amount of sample was placed into the holder and scanned in the range of 2θ=5-45°.
Swelling capability: The water sorption capacity was carried out using two different phosphate buffer solutions (pH 2.5 and 6.8) as immersion media (N=3) [29]. Initially, each tablet was carefully weighed (W1) and then soaked in two different phosphate buffers, pH 2.5 and 6.8, respectively. The tablet remnants were wiped off any excess surface water using a filter paper and weighed (W2) at different time intervals. In order to measure the water sorption, the following equation was used:
% water sorption=(W1-W2)/W2 × 100….(1)
Water contact angle: The water contact angle was measured using an Ossila L2004A1 contact angle goniometer at 25°C. The contact angle was determined by carefully placing a water droplet (5 μL) on the surface of the samples. Three measurements were performed, and the average angle was measured. All results were analyzed using the Ossila Contact Angle software.
Cytotoxicity study-MTT Assay: To investigate the biocompatibility of the newly synthesized chitosan-derivatives (CS, CS-OCNC, CS-ODEX), the materials were incubated for 1, 3 and 5 days with a primary cell line of Human Periodontal Ligament Cells (hPDLCs), isolated as previously described [30]. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed for different material concentrations (C1=0.125 mg/ml, C2=0.25 mg/ml, and C3=0.5 mg/ml). All the materials were sterilized for 30 minutes under UV light. 103 cells per well were seeded overnight in 96-well plates for 24 h in a 37°C sterile incubator. The next day, the materials were added in quadruplicates and incubated for 1, 3 and 5 days. Cells (positive control without materials) and cells with the materials were cultured with the conventional medium (DMEM, fetal bovine serum 10%, and Penicillin/Streptomycin (P/S) 1%). After each incubation time, the MTT solution was added for 3 hours, then the supernatants were discarded and DMSO was added to dissolve formazan crystals for 30 min. After that time the absorbance was measured spectrophotometrically at 540 and 630 nm using an Elisa microplate reader (Thermofischer Scientific).
In vitro drug release: The dissolution experiments were carried out in a USP type II apparatus (Pharmatest, series type PT-DT7) in aqueous medium (pH=6.8, 450 mL). The tested matrix tablets were placed in beakers under sink conditions at 50 rpm. Samples were removed and filtered at predetermined time intervals and analyzed using a UV spectrophotometer (LLG Labware, series type uniSPEC 2), at λmax=301 nm.
As previously mentioned, the main aim of the present study was to evaluate the use of CS-based derivatives, with an abundance of –OH groups grafted in their macromolecular backbone chains, in the fabrication of the tablets. The hydroxyls can enhance the hydrophilicity of a material, and thus have a direct impact upon its swelling and, consequently, to the mucoadhesive properties. Figure 1 presents an optical observation of the prepared CS tablets using a microscope. It is obvious that in all cases, smooth and without cracked surfaces tablets have been successfully prepared. These images show the potential of the prepared CS derivatives as well as of the selected excipients in the current work towards the effective preparation of drug release tablets (Figure 1).
The produced tablet formulations were characterized in terms of structure and cytotoxicity, and the structure-properties relationships, focusing on swelling and hydrophilicity (and subsequently their mucoadhesive behavior). Moreover, the in vitro release of OME from the developed matrix tablets, was studied, and a release mechanism is proposed.
Investigation of the drug-polymer interactions
ATR-FTIR analysis was performed upon all formulations in an attempt to decipher the CS-OME interactions, by observing peak shifts, alterations in their intensity, the appearance of new absorbance bands, or any other indications that may imply any possible drug-polymer matrix interactions (Figure 2).
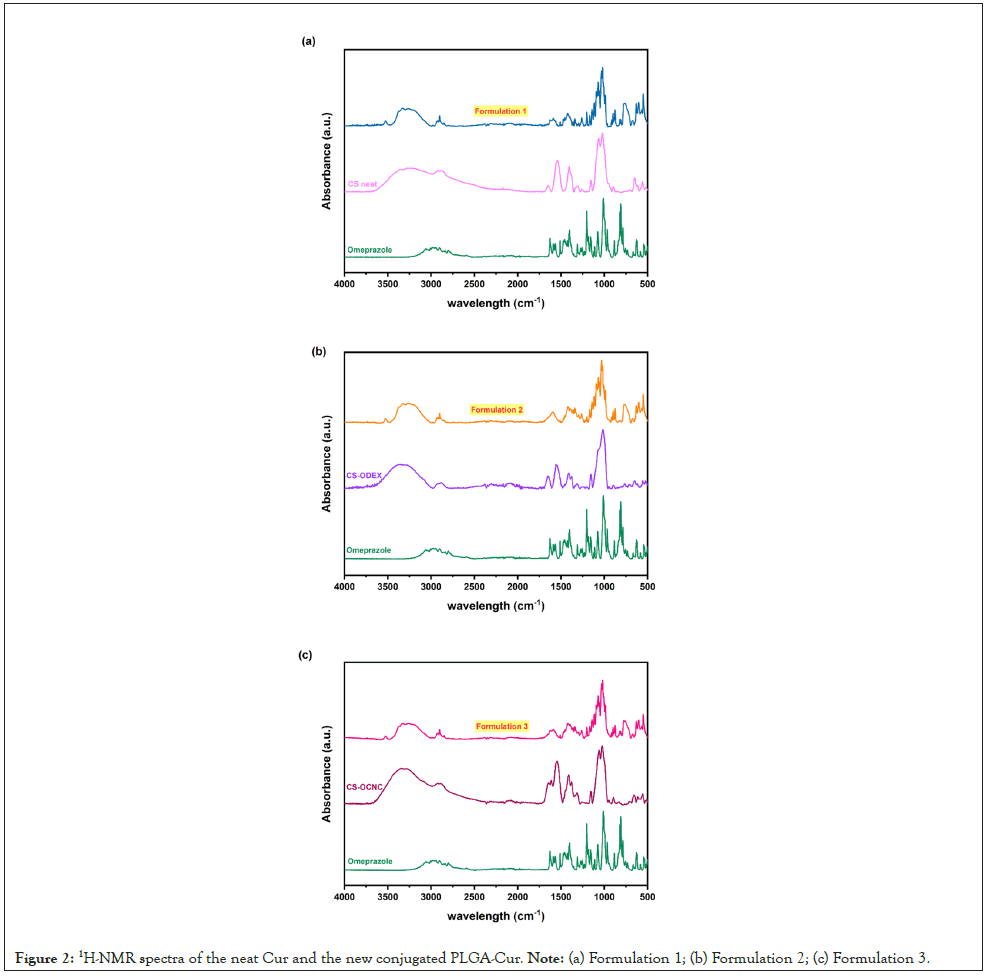
Figure 2: 1H-NMR spectra of the neat Cur and the new conjugated PLGA-Cur. Note: (a) Formulation 1; (b) Formulation 2; (c) Formulation 3.
As it can be seen, the OME’s spectrum demonstrates several characteristic bands, especially the N-H stretching vibration at 3352 cm-1, the C=N bond stretching vibration band at 1627 cm-1, the N-H bending at about 1405 cm-1, the S=O stretching at 1070 cm-1, and the asymmetric CO-C stretching bands at about 1203 cm-1 [31].
The main bands of CS are located at 3464 and 3228 cm-1 (-NH2- OH stretching, respectively), 2922 and 2875 cm-1 (C-H symmetric and asymmetric stretching), 1659 cm-1 (>CO stretching-amide I), 1542 cm-1 (N-H bending of the primary amines), 1413 cm-1 (C-H and O-H vibrations), 1148 cm-1 (anti-symmetric stretching of the C-O-C bridge) and 1066 cm-1 (skeletal vibrations involving the C-O stretching) (Figures 2a-2c) which are characteristics of its polysaccharide structure [32,33]. In the case of the modified chitosan derivatives (Figures 2a and 2b), a broadening of the -OH stretching vibrations band associated with the incorporation of hydroxyl groups and the appearance of a new peak at 1647 cm-1, attributed to the presence of the C=O stretching of the amide I band, were observed. Moreover, an increment of the peak intensities at ~1350 and ~1160 cm-1, assigned to the C-H bending of the aromatic esters and the anti-symmetric stretching of C-O-C bridge, respectively, are both indications of the successful modification, resulting from the integration of the dextran (CS-ODEX) and cellulose (CS-OCNC) aromatic moieties onto the chitosan backbone.
The ATR spectra of the prepared formulations did not exhibit any further shifts that could imply drug-polymer matrix interactions, and the very characteristic peak of the sulfoxide (S=O) stretching vibration of OME at 1070 cm-1 (Figures 2a-2c), although weak, due to the small amount of drug added, appeared at the same position. The recorded peaks were mainly dictated by the characteristic bands of the tablet excipients that constitute the largest fraction of the sample.
Evaluation of crystalline structures
To verify the physical structure of the drug inside the tablet, XRD measurements were performed, and the recorded patterns were analyzed in comparison to the neat materials (Figure 3). Omeprazole is a pharmaceutical compound in crystalline form with several characteristic peaks (9.09°, 11.03°, 12.15°, 17.25°, 23.63°, 27.12°, as well as several others of much lower intensity) (Figure 3a). Similarly, some crystallinity is observed in the case of the polymers used as excipients during tableting (except the sodium alginate, which is completely amorphous), and their characteristic peaks indicated on the patterns (Figure 3b), mainly appearing in the region of 2θ=19- 22 ο.
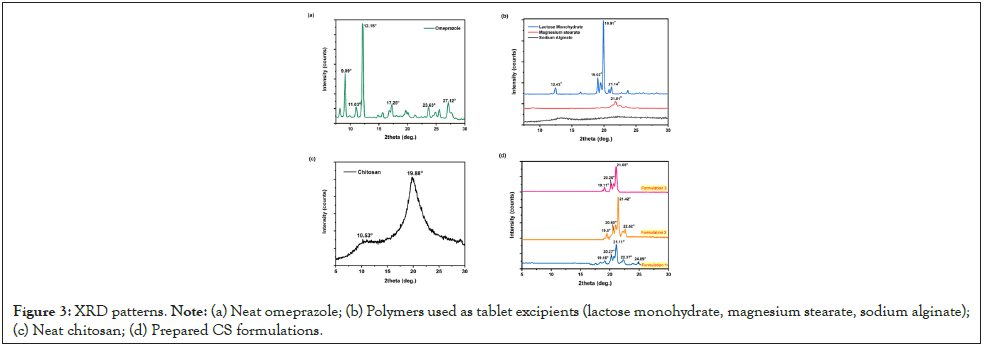
Figure 3: XRD patterns. Note: (a) Neat omeprazole; (b) Polymers used as tablet excipients (lactose monohydrate, magnesium stearate, sodium alginate); (c) Neat chitosan; (d) Prepared CS formulations.
On the other hand, CS is a semi‐crystalline polymer giving two broad halo peaks at 2θ=10.53 and 19.88° (Figure 3c). This form is known to be related to the strong hydrogen bond formation between chitosan's hydroxyls, but also between its amino and hydroxyl groups. In a series of previous works, our group reported that the introduction of side groups in the CS’s macromolecular chains after modification, reduces their folding ability and thus prevents the generation of crystallites, which usually leads to less crystalline or completely amorphous derivatives [34-36]. Looking closely at the obtained patterns of the prepared formulations, this factor is repeated in this case, as well. Although in all samples the most prominent peaks are the ones attributed to the crystalline excipients (lactose monohydrate, magnesium stearate), which, as already mentioned, are abundant in the formulations, in the case of the neat CS-based sample, more peaks are recorded in the region of interest (19-22 ο), thus implying a structure of higher crystallinity.
The characteristic crystalline peaks of OME at 9, 11 and 12 ο (Figure 3a) disappear in the diffractograms of the prepared formulations (Figure 3d). This finding suggests the amorphous dispersion of OME inside the tablets, which is highly desirable in drug delivery formulations leading to increased solubility and faster dissolution rates. Furthermore, amorphous drugs may exhibit increased chemical stability that can extend the shelf-life of drug products, improve bioavailability, and lead to higher drug concentrations in systemic circulation and improved therapeutic outcomes (Figure 3) [37].
Swelling behavior studies
The first step in the mucoadhesion process involves the polymer wetting and swelling, succeeded by the interpenetration and entanglement of polymer chains with mucin residues, leading to the creation of hydrogen bonds [38]. Water-soluble polymers with bioadhesive behavior, such as sodium alginate and cellulose derivatives, are frequently used in this type of formulations. The optimal polymer employed in mucoadhesive formulations should be non-toxic and non-irritating, and it should also exhibit minimal absorption from the gastrointestinal tract. Moreover, it must form a strong non-covalent bond to the surface of the mucin epithelial cells and adhere to most tissues. The polymers used in these dosage forms should have the appropriate physicochemical properties, such as high hydrophilicity and the presence of groups capable of forming hydrogen bonds [11].
The swelling behavior is maybe the most crucial factor for an efficient and prolonged adhesion of the material upon the mucus surface. It has already been reported that the mucoadhesive strength of a cellulose derivative depends on its water uptake capacity [39]. Higher values imply a more stable formed gel, and enhanced interactions. For this reason, the swelling behavior of all formulations was studied at two different pH values, 6.8 and 2.5 (simulating the acidic lower stomach environment).
As it can be seen in Figure 4, the comparison of the swelling behavior of all three prepared formulations showed similar water sorption profiles and rates. In particular, they reached their maximum swelling degree within the first two hours, although no further swelling was detected up to 5 h.
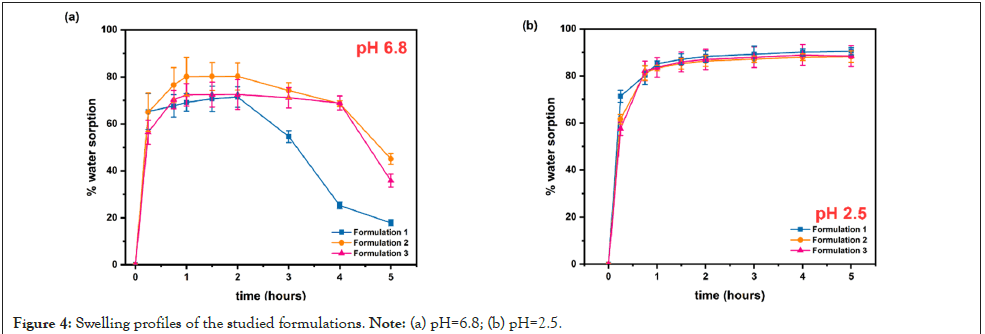
Figure 4: Swelling profiles of the studied formulations. Note: (a) pH=6.8; (b) pH=2.5.
Khare et al., reported that the presence of sodium could significantly increase the water uptake of polymers and according to Mortazavi et al., the ability of a polymer to take up water from mucus is a primary determinant of potential mucoadhesive behavior [40,41]. In our previous works, we have reported that the addition of carboxyl groups leads to a more hydrophilic derivative and a higher degree of swelling than in the case of neat CS [36,42,43]. This is also evident in the present study, especially when the test was performed in pH 6.8 buffer medium, with the modified chitosanbased tablets achieving higher rates than the neat one, before the network collapsed (~ after 3 hours of study) (Figure 4a). What is even more significant, though, is the substantial increment of % water sorption, and thus of system stability, when the test was performed under acidic conditions (Figure 4b).
The improved swelling effect, in the case of the acidic medium, was also verified by the appearance of the samples when the relevant experiments were through. A compact and still stable network was obtained after the multiple immersions in PBS, pH 2.5 (Figure 5a), whereas the cohesive forces of the sample network collapse early when the swelling is performed at pH 6.8, leading to a gradual disintegration of the hydrogel (Figure 5).
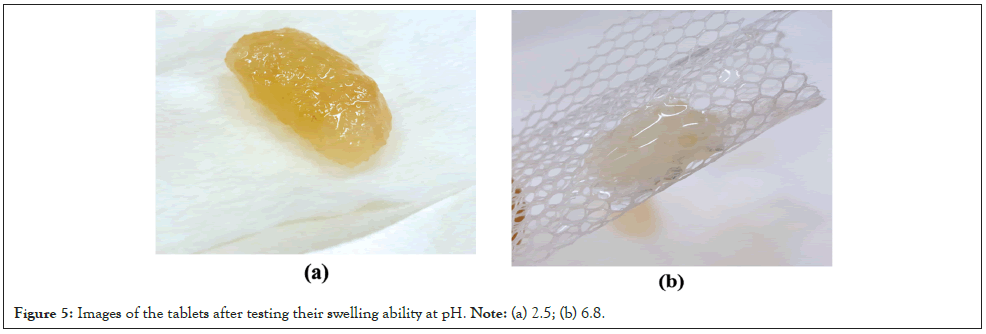
Figure 5: Images of the tablets after testing their swelling ability at pH. Note: (a) 2.5; (b) 6.8.
Water contact angle
The mucoadhesive behavior of a range of natural and synthetic polymers has been investigated via several techniques [44,45]. Sodium alginate has been identified as one of the top-ranking mucoadhesive polymers. Miyazaki et al., after studying the bioadhesion property of CS/alginate tablets with different mixing ratios (1:4, 1:1 and 4:1) found that the adhesion force decreased with a decrease of the alginate content in the tablets [21].
The interaction between the prepared formulations and wet media, and thus a prediction of a potential mucoadhesive behavior, when in contact with an underlying tissular environment, was assessed by measuring the hydrophilicity of the tablets via determining the static water contact angle. The contact angle indicates the degree of wetting when a liquid (i.e., the adhesive candidate) and a solid (i.e., the mucosa) interact with each other. A contact angle with a value equal or close to zero implies an adequate spreadability of the adhesive candidate onto the mucosal tissue, which is a prerequisite for the mucoadhesion [46-48].
In Figure 6 the measurement results for the three formulations, up to 60 sec, is depicted. Chitosan is a cationic polymer and its adhesion to mucus mainly derives from the ionic interactions with the anionic substructures of mucus layer [49]. The incorporation of hydrophilic groups, such as -OH moieties, through chemical modification in the CS molecule could further increase its adhesion capability. The contact angles measured at t0 were 77.58, 73.12, and 54.93˚ for neat CS, CS-ODEX, and CS-OCNC, respectively. As it is revealed through the successively captured images at different timepoints, CS-OCNC demonstrated the strongest hydrophilic surface, reaching a contact angle close to 0˚ in less than t=5 sec, demonstrating a strong swelling effect. Analogously, the CSODEX sample shows enhanced hydrophilicity compared to the neat chitosan-based formulation. The swelling effect observed in both derivatives, after 60 sec, additionally suggests a stable to water uptake system (Figure 6).
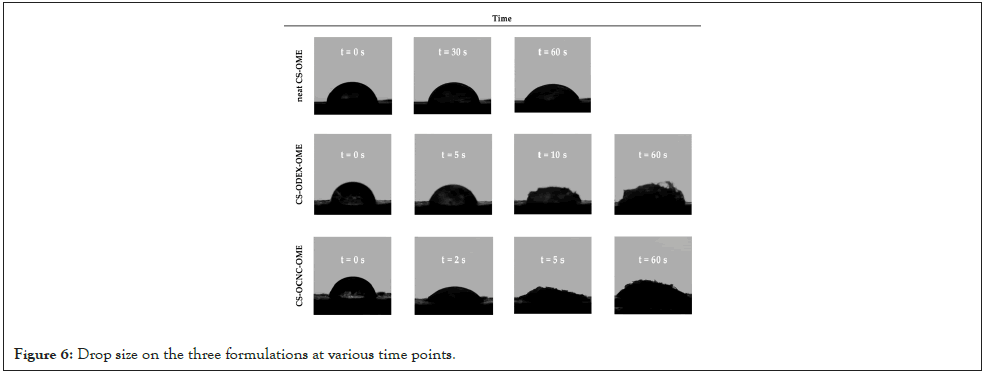
Figure 6: Drop size on the three formulations at various time points.
Cell viability
The biocompatibility of CS has been widely investigated in the literature and thus chitosan has been proposed very frequently as a carrier for the delivery of active agents [50]. Interestingly enough, previous research has showed that CS’s degradation products, such as chito-oligosaccharides, may stimulate Schwan cell proliferation and accelerate the cell cycle. Its chemical modification with hydrophilic compounds, such as ODEX and OCNC may result to further enhancement of its inherent biocompatibility [51].
The investigated samples were compared to the control groups by performing the MTT assay, as described and detailed in the Materials and methods section. The biocompatibility assay of the tested chitosan-derivatives (CS, CS-OCNC, CS-ODEX) revealed a non-toxic behavior at all the tested time points. In detail, when cells were incubated with neat CS there was no difference of cell proliferation compared to the control cells, suggesting the absence of toxicity. In the case of the modified samples, CS-ODEX presented the most abundant increase of cell proliferation, especially after 3 and 5 days of incubation at all concentrations. Regarding the CS-OCNC derivatives, although there was a slight decrease on day 3, compared to day 1, the cells presented a significant increase in cell proliferation after 5 days. All tested concentrations exhibited greater than 70% cell viability over a 120 h period. These results indicate that high cell viability (>70%) and metabolic activity were maintained. According to the International Organization for Standardization, part 5: Tests for in vitro cytotoxicity of medical devices (ISO 10993-5:2009 guidelines), all chitosan samples are considered to be non-cytotoxic, with the CS-ODEX derivative promoting cell proliferation even at the highest concentration (Figure 7) [52].
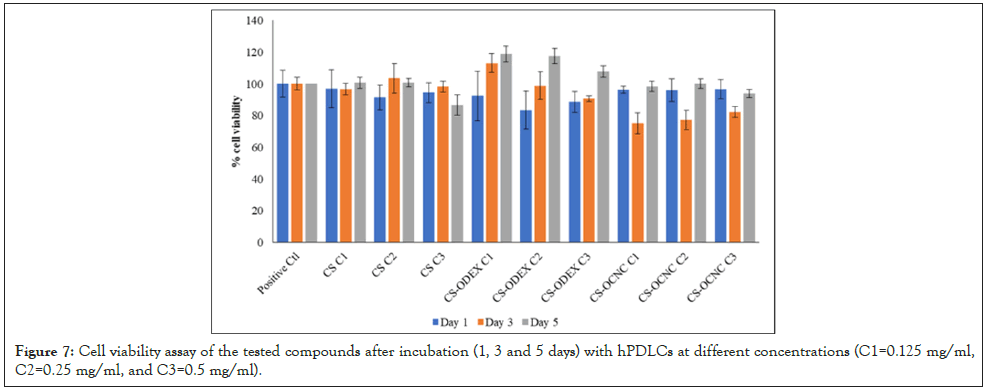
Figure 7: Cell viability assay of the tested compounds after incubation (1, 3 and 5 days) with hPDLCs at different concentrations (C1=0.125 mg/ml, C2=0.25 mg/ml, and C3=0.5 mg/ml).
In vitro release of omeprazole from CS tablets
As already mentioned, omeprazole suppresses stomach acid secretion by specific inhibition of the H+/K+-ATPase system found at the secretory surface of gastric parietal cells. Because this enzyme system is regarded as the acid (proton, or H+) pump within the gastric mucosa, omeprazole inhibits the final step of acid production [53]. These inhibitory effects of omeprazole occur within 1 hour after oral administration, whilst its absorption takes place in the small intestine and is usually completed within 3 to 6 hours [54].
In the present study the derived % dissolution vs. time curves, depicted in Figure 8, reveal that omeprazole’s release from the developed matrix tablets formulations, 2 and 3, each containing 13 mg of oxidized dextran (CS-ODEX) and oxidized nanocellulose (CS-OCNC), respectively, reaches 77.7%, at t=180 min, and 83.7%, at t=240 min. Thereafter, and until the end of the experiment (t=300 min), OME’s release follows a very similar trend. These findings are in alignment with the OME’s characteristic inhibitory effects, mentioned above, and almost within the same time framework. Conversely, a slower release pace is showed in the case of the matrix tablets, comprising of neat chitosan (Formulation 1) (Figure 8). Apparently, at pH=6.8, the -NH2 groups on the neat chitosan’s structure (at C2) are non-protonated to account for its high solubility, observed in acid aqueous media. As a result, CS’s % release, at t=180 min, is 46.7, at t=240 min, 57.6, and at t=300 min, the OME’s % release rises to 75.6. Albeit the fact that the release of omeprazole is slower from the neat CS matrix tablets than from the (CS-ODEX) and (CS-OCNC) formulations, the linearity observed in its % release vs. time pattern satisfies the sought inhibitory effects (Figure 8).
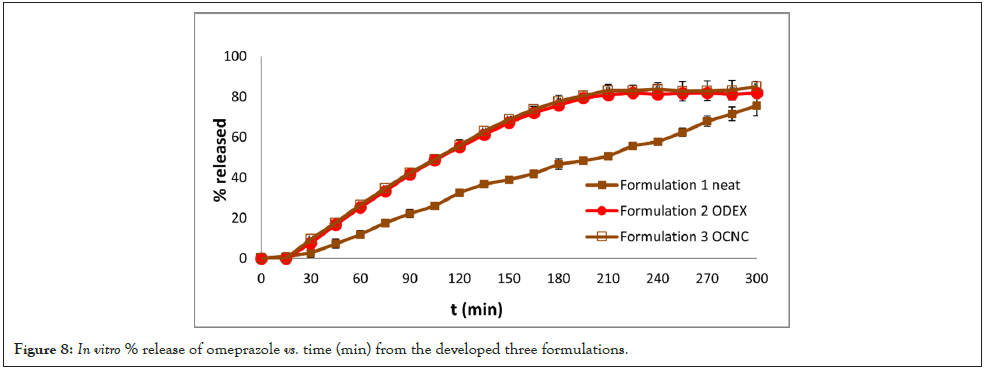
Figure 8: In vitro % release of omeprazole vs. time (min) from the developed three formulations.
Modeling of drug release data
The release of drugs from hydrogels is a quite complex process. The first step includes the diffusion of water inside the hydrogel leading to its swelling. The diffusion coefficient of water is, in general, a function of the local water concentration. As the water diffuses there is a competition between the diffusion and the polymer chain relaxation processes, which correspond to a time delay between the contact of the polymer with the water and the swelling. The drug is initially immobilized in the dry polymer. As the water approaches the drug, it becomes mobile and free to diffuse. The drug diffusivity is also a function of the local water content. In addition to the above phenomena, erosion of the external swollen layers of the polymer matrix takes place. This affects the immediate release of the drug from the eroded matrix. The mathematical problem is difficult to be solved since it is defined by two partial differential equations for diffusion (water and drug) in a domain with moving boundaries. Three fronts are created: The first front is the one that separates the dry from swollen polymer matrices, the second front is created by the drug and the third is the outer layer of the matrix undergoing erosion [55]. There are many literature reports for the simulation of drugs release from hydrogels [56,57]. In one of their works, Wu et al., implemented diffusion equations for water and drug and volume expansion (swelling) based on a global mass balance for the diffused water and drug [58]. In addition, the erosion process was considered. The diffusivities are assumed to be functions of the water content. However, the model does not consider the relaxation of the polymer chains and assumes a uniform expansion. This may be a problem since the local expansion depends on the local concentration of water. In order to avoid the mathematical complexity of the problem several simplified approaches have been proposed. In the case of slow drug diffusion with respect to polymer relaxation time the problem can be assumed as a purely diffusive one with a t0.5 time dependence of the released drug, for small times and an exponential (first order) dependence for large times. In the other limit of slow polymer relaxation compared to drug diffusion, the so-called Case II transport results. It corresponds to a zero-order kinetic process with a linear dependence with time of drug released. Comparable relaxation and diffusion times lead to anomalous transport with the time exponents’ values ranging from 0 and 1 [59]. Another limiting case is the erosion dominated drug release. The kinetics process for the slab geometry is also of zero order [60]. According to the above, a zero-order kinetic process does not allow the selection of a dominant mechanism between Case II transport or erosion, without any additional information being available.
With respect to the release process involved in the present study, two steps are involved. The release of drug to the region of liquid adjacent to the polymer matrix and the mass transfer from this region to the bulk of the liquid through convective diffusion is the first step. The second step must be significantly faster than the first considering the time scale of the release and the intense agitation of the bulk liquid. Thus, the drug release is completely determined by the polymer matrix scale problem. With respect to formulation 1, the release curve can be assumed to consist of two parts: (i) A small delay probably due to the time needed by the water to relax the polymer chains and to mobilize the drug, and (ii) A linear part corresponding to the zero-order model of release. The swelling is much faster than the release, leading to the conclusion that the zero order of the release dynamics is due to the erosion of the polymer matrix. The release equation is given as R=0 for t ≤ T and R=α (t-T) for t>T, where R is the percentage of the drug released. The values resulted from the fitting are T=12 min, α=0.26 min-1. The comparison between the fitting curve and the experimental details is demonstrated in Figure 9. The determination of the R2 coefficient of the fit is larger than 0.99. The formulations 2 and 3 lead to practically the same release curve. The existence again of a delay time is quite obvious. The approach to an asymptotic value creates suspicions about the domination of diffusion. However, attempts to fit the data with exponential (first order model) curves corresponding to diffusion failed and so did the powerful tool of double exponentials. The above models are related to diffusion, which is obviously absent in the present case. It appears that a zeroorder model has a fair success in fitting the data up to the plateau value. Interestingly enough, adding a second order term to the zeroorder model leads to a quite accurate fit of the data (R2>0.999). The fitting curve has the form R=0 for t<T1, R=α (t-T1)2+β(t-T1)2 for T1 ≤ t ≤ T2, R=F for t>T2. The constants in the equation for formulations 1 and 2, are T1=17, T2=210, α=0.66 min-1, β=-0.00121 min-2, F=82 and T1=17, T2=210, α=0.677 min-1, β=-0.00126 min-2, F=84 respectively.
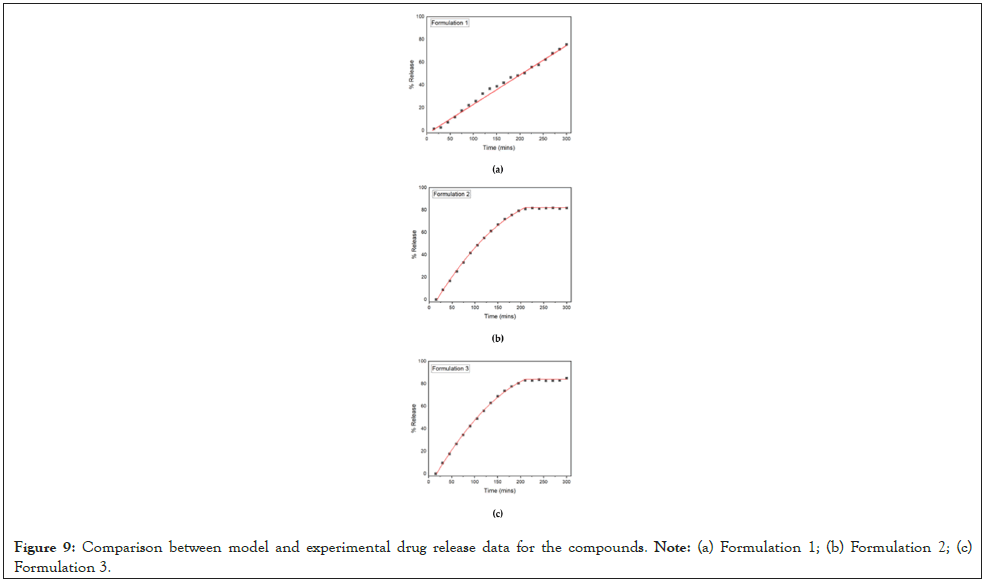
Figure 9: Comparison between model and experimental drug release data for the compounds. Note: (a) Formulation 1; (b) Formulation 2; (c) Formulation 3.
The prevalence of the lineal term suggests that the release mechanism is erosion. It appears that such an equation has never been used to describe the release process. Seemingly it belongs to the class proposed by Peppas et al., with R=K1tm+K2t2m [61]. However, according to this study, m must be smaller than ½, whereas in the present case it takes the value m=1. The attempt to fit the data with m=½ led to failure. The comparison between fitting curves and experimental data appears in Figure 9.
This study successfully demonstrated the potential of chitosan-based derivatives (CS-ODEX and CS-OCNC), modified with hydrophilic hydroxyl groups, as efficient biopolymers for the development of drug release tablet formulations. The structural integrity of the tablets was confirmed by smooth, crack-free surfaces, while their swelling ability and mucoadhesive properties were evaluated. The formulations showed significant swelling behavior, particularly in the acidic environments, indicating enhanced mucoadhesion. Drug-polymer interactions, as evaluated by ATR-FTIR and XRD studies, revealed the amorphous dispersion of omeprazole within the polymer matrices, which is critical for improved drug solubility and dissolution rates. This amorphous state is particularly favorable for enhancing bioavailability and prolonging drug release, as evidenced by the consistent and controlled in vitro release profiles observed in the CS-ODEX and CS-OCNC matrices compared to neat CS formulations. The hydrophilicity of the modified CS derivatives, as indicated by the water contact angle measurements, justifies their superior mucoadhesive behaviour. Furthermore, the biocompatibility of these derivatives was confirmed through the MTT assay, with no cytotoxic effects observed. Notably, the CS-ODEX derivative promoted cell proliferation, enhancing its suitability for drug delivery applications. In summary, the incorporation of hydrophilic moieties into chitosan significantly improved its swelling, mucoadhesive and drug release properties, making CS-ODEX and CS-OCNC promising candidates for mucoadhesive drug delivery systems, promoting a gastro-resistant modified release. Further studies will be needed in order to explore the in vivo performance and long-term stability of these materials.
Methodology, investigation, formal analysis, N.D.B; E.C; R.B; in-vitro release investigation, formal analysis, I.S; A.S; release modeling, writing, M.K; cytotoxicity studies, writing, I.T; writingoriginal draft preparation, review and editing, N.D.B; E.C; supervision; E.K; conceptualization, supervision; P.B; supervision, writing-review and editing, M.V.
There is no conflict of interest between the authors.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Bikiaris ND, Barmpalexis P, Christodoulou E, Bikiaris R, Kostoglou M, Tsamesidis I, et al. (2024). Fabrication of Mucoadhesive Chitosan-Based Omeprazole Matrix Tablets: In Vitro Modified Release Studies. J Appl Pharm. 16:440.
Received: 22-Oct-2024, Manuscript No. JPMR-24-34753 ; Editor assigned: 24-Oct-2024, Pre QC No. JPMR-24-34753 (PQ); Reviewed: 11-Nov-2024, QC No. JPMR-24-34753 ; Revised: 19-Nov-2024, Manuscript No. JPMR-24-34753 (R); Published: 27-Nov-2024 , DOI: 10.35248/1920-4159.24.16.440
Copyright: © 2024 Bikiaris ND, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.