Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2018) Volume 7, Issue 3
Keywords: Knoevenagel condensation; Aldehydes; Arylidene acrylate; Ethylcyanoacetate; Ammonium acetate; Solvent free;Microwave irradiation
Many chemical processes employ large amount of hazardous and toxic solvents. The choice of pursuing aqueous reactions is becoming more important and urgent than ever before, due to its environmental impact and cost of chemical processes [1]. Organic reactions under solvent free conditions have increasingly attracted chemists’ interests particularly from the viewpoint of green chemistry [2-4]. The Knoevenagel condensation between aldehyde and active methylene compounds is an important reaction in organic synthesis in forming C=C double bonds. It has been used for the preparation of a range of substituted alkenes and bioactive molecules and as a key step in natural product synthesis. In general, the Knoevenagel condensation is carried out using organic bases such as aliphatic amines, urea, ethyllenediamine and piperidine or their corresponding ammonium salts, and the amino acids glycine [5,6]. Among these catalysts the ammonium salt such as NH4OAc is another inexpensive, available, efficient and mild catalyst for the synthesis of Knovenagel condensation of arylidine derivatives. The Knovenagel condensation has been receiving considerable attention, due to its broad spectrum of uses including cosmetic, perfume, agrochemicals and pharmaceuticals application [7]. In the last ten years, a lot of methods to achieve Knoevenagel condensation are known. For example, some novel heating methods such as microwave irradiation, had been applied in Knovenagel condensation [8]. Meanwhile, accumulating efforts have been made to explore novel catalysts for Knovenagel reaction [9,10]. However, some of these methods suffer from some drawbacks such as long reaction time, the uses of harmful catalyst, lots of solvent and energy expense. Microwave promoted organic synthesis is a fast, efficient method of heating a system in order to achieve the completion of a desired reaction. Microwave promoted organic synthesis can help to produce high yields of products in a short time, while reducing side reactions and making reaction easier. The syntheses of some arylidenemalonitriles by the reaction of malonitriles and aldehydes as well as synthesis of selective acrylate by condensation reaction have also been reported [11,12].
The present work, therefore, is to synthesis of Knoevenagel condensation of various aromatic aldehydes with active methylene compounds using NH4OAc as catalyst under solvent free condition.
All chemicals were received from commercial sources (Sigma- Aldrich and Merck, Germany) and were used without further purification.
Synthesis and characterization of acrylates
The reaction was carried out in a 50.0 mL beaker in which an equimolecular mixture of aromatic aldehyde 1 and active methylene compound 2 (1:1) were added in a catalytic amount of ammonium acetate (NH4OAc). All the reaction scales were same, such as 2.0 mmol of each starting material and 0.25 mmol catalyst. For example, a mixture of 4-bromobenzaldehyde (2.0 mmol), ethyl cyanoacetate (2.0 mmol) and ammonium acetate (0.25 mmol) was placed in a pyrex glass 50.0 mL beaker. The reaction mixture was mixed thoroughly with the help of a glass rod. The mixture was then subjected to microwave irradiation at 300-800 W for the reaction period of 1∼3 minutes. The progress of the reaction was followed by thin layer chromatography (TLC) (hexane:ethyl acetate, ratio:4:1). After completion of the reaction, it was cooled and then the solid mass of the products was recrystallized using ethyl acetate and n-hexane solvent mixture. The reaction process is shown in Scheme 1.
All the compounds showed a single spot on the TLC plate. From these observations, therefore, it was suggested that the compounds were almost 99.0% pure.
Gallen kamp melting point apparatus was used to record the melting point of the solid compounds, the heating was done carefully to ensure a steady rise of temperature. The TLC was performed on Kieselgel GF254 and visualization was accomplished by UV light. Infrared spectra were observed on a Shimadzu FTIR- spectrometer (IR prestige 21). 1H NMR spectra were recorded on a JEOL JNM ECS FT NMR at 400 MHz spectrometer using TMS as an internal standard. Coupling constants J are given in Hz. The mass spectra were recorded on a Jeol SX-102 (FAB) spectrometer. Analytical TLC was performed on pre-coated alumina sheet and the product was visualized by UV light. All the reactions were carried out in a domestic microwave oven (Samsung, MW76ND).
Antibacterial activity assay
The antibacterial activity assay was performed by disc diffusion method [13] with little modification against two Gram-negative bacteria such as: (i) Klebsiella oxytoca and (ii) Escherichia coli at the concentration of 60-200 μg/disc, which is a qualitative to semi quantitative test. Briefly, 6.0 mm diameter and 1.0 mm thickness normal Whitman filter paper no.1 was used as sample disc. The compounds (2.0 mg) to be tested were dissolved in methanol (2 mL). The concentration used in this study was 1.0 mg/mL of each sample. The completely dried filter paper discs were placed on the surface of the seeded agar medium in triplicate tests for each sample. Plates were allowed to stand for 2 h for complete diffusion. Then, the plates were incubated at 37°C for 24 h to 48 h, after which the susceptibility of each organism to each sample was estimated by measuring the diameter of the zones of inhibition. The plate with sample and antibiotic was kept in incubator and observed the result after 24 h. Sometimes it may necessary to keep it for 48 h. Gentamycin was used as the standard. The results of the activity test are shown in Table 1.
| Compounds SL no. | Compounds name | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Klebsiella oxytoca | Standard | Escherichia coli | Standard | ||
| 3a | Ethyl-2-cyano-3-(4-bromophenyl) acrylate | -- | -- | 6 | 11 |
| 3b | Ethyl-2-cyano-3-(4-fluorophenyl) acrylate | 6 | 7 | -- | -- |
| 3c | Ethyl-2-cyano-3-(4-pyridinphenyl) acrylate | 6 | 17 | 7 | 27 |
| 3d | Ethyl-2-cyano-3-(4-methylphenyl) acrylate | 6 | 6 | 8 | 25 |
| 3e | 2-(4-fluorobenzylidene)-1-phenylbutane-1,3-dione | 5 | 6 | -- | -- |
| 3f | Methyl-2-(4-fluorobenzylidene)-3-oxobutanoate | 6 | 6 | 7.5 | 25 |
| 3g | Methyl-2-(4-nitrobenzylidene)-3-oxobutanoate | -- | -- | -- | -- |
| Key: “--” means inactive against used bacteria. | |||||
Table 1: Antibacterial activity for the synthesized compounds. Gentamycin was used as standard.
Characterization
In general, the reactions were clear and free from the Michael adduct. All the products were characterized by 1H-NMR, 13C-NMR, IR and Mass spectroscopic data analyses. The 1H NMR spectra of the products 3a-3i showed the olefinic proton around δ 7.5-9 ppm (in CDCl3) as singlet peak. In the IR spectrum, the absorption peak observed at 1703-1727 cm–1 due to the carbonyl, and the band at 2213-2221 cm–1 is due to the -CN groups. Our results are in good agreement with reported value [14-16]. The spectroscopic data of all the products are given below sequentially. All the FT-IR, 1H-NMR, 13C-NMR, and Mass spectroscopic data are given as supporting information (SI).
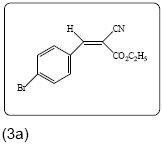
Ethyl 2-cyano-3-(4-bromophenyl) acrylate (3a): Yield 87% (322.0 mg), white crystals, Rf=0.68 (in EtOAc/Hexane: 0.5:4.5), (99.0% pure), Mp: 90∼92°C. IR (KBr) υmax(cm–1): 3031 (aromatic=C–H), 2985 ( alkene=C–H) 2221 (C ≡ N), 1719 (C=O), 1611 (C=C). 1H NMR δppm 8.17 (s, 1H), 7.86 (d, 2H, J=8.8 Hz), 7.65 (d, 2H, J=8.4 Hz), 4.37(q, 2H, J=7.2 Hz), 1.39 (t, 3H, J=7.2 Hz). 13C NMR (CDCl3) δ ppm 162.1, 153.4, 132.6, 132.2, 130.2, 128.2, 115.2, 103.6, 62.8, 14.1. Mass (m/z): 280, 154, 137, 89, 39. MF=C12H10O2BrN.
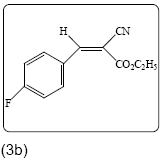
Ethyl 2-cyano-3-(4-fluorophenyl) acrylate (3b): Yield 82% (203.0 mg), Rf=0.87 (in EtOAc/Hexane: 1:4), white crystals, (99.0% pure), Mp: 80∼82°C. IR (KBr) υmax(cm–1): 3108 (aromatic=C–H), 2993 (alkene=C–H), 2845, 2221 (C ≡ N), 1719 (C=O), 1611 (C=C). 1H NMR δppm 8.19 (s, 1H), 8.01 (m, 2H, J=5.6 Hz), 7.18 (m, 2H, J=8.4 Hz), 4.37 (q, 2H, J=7.6 Hz), 1.38 (t, 3H, J=6.8 Hz). 13C NMR (CDCl3) δ ppm 162.3, 134.2, 134.1, 132.4, 127.8, 117.7, 117.0, 116.4, 62.7, 14.0. Mass (m/z): 220, 137, 136, 89, 39. MF=C12H10O2FN.
Ethyl 2-cyano-3-(4-pyridinphenyl) acrylate (3c): Yield 96% (206.0 mg), Rf=0.68 (in EtOAc/Hexane: 0.5:4.5), brown crystals, (99.0%pure), Mp: 175∼177°C. IR (KBr) υmax(cm–1): 3039 (aromatic C–H), 3000 (alkene=C–H), 2221 (C ≡ N), 1727 (C=O), 1619 (C=C). 1H NMR δppm 8.18 (s, 1H), 8.81 (d, 2H, J=6.4 Hz), 7.74 (d, 2H, J=6.4 Hz), 4.41 (q, 2H, J=7.2 Hz), 1.41 (t, 3H, J=7.2 Hz). 13C NMR (CDCl3) δ ppm 161.2, 152.0, 151.0, 138.0, 123.2, 114.2, 108.3, 63.3, 14.0. Mass (m/z): 203, 175, 131, 89, 39. MF=C11H10O2N2.
Ethyl 2-cyano-3-(4-methylphenyl) acrylate (3d): Yield 96% (231.0 mg), Rf=0.48 (in EtOAc/Hexane: 1:4), white powder, (99.0% pure), Mp: 88∼90°C. IR(KBr) υmax(cm–1): 3031 (aromatic C–H), 2993 (alkene=C–H), 2213 (C ≡ N), 1727 (C=O), 1600 (C=C). 1H NMR δppm 8.30 (s, 1H), 7.93 (d,2H, J=8.4 Hz), 7.37 (d, 2H, J=8.4 Hz), 4.29 (q, 2H, J=7.2 Hz), 1.28 (t, 3H, J=7.2 Hz), 2.35 (s,3H). 13C NMR (CDCl3) 162.5, 155.4, 144.9, 131.5, 130.5, 130.0, 129.2, 116. 6, 21.8, 14.5. Mass (m/z): 216, 170, 137, 91, 43. MF=C13H13O2N.
2-(4-fluorobenzylidene)-1-phenylbutane-1,3-dione (3e): Yield 95% (236.0 mg), Rf=0.75 (in EtOAc/Hexane: 1:4), light yellow crystal,(99.0% pure), Mp: 108∼110°C. IR(KBr) υmax(cm–1): 3101 (aromatic C–H), 2946 (alkene=C–H), 2840 (–CH3), 1703 (C=O), 1649 (C=C). 1H NMR δ ppm 7.75 (s, 1H), 7.90 (m, 2H), 7.55 (m, 1H), 7.41 (m, 2H), 7.33 (m, 2H), 7.91 (m,2H), 2.37 (s, 3H). 13C NMR (CDCl3) δ ppm 197.9, 195.5, 164.9, 162.4, 139.7, 139.2, 135.8, 134.2, 132.4, 132.3, 129.1, 129.0, 116.1, 115.9, 27.2. Mass (m/z): 269, 105, 77, 43. MF=C17H13O2F.
Methyl 2-(4-fluorobenzylidene)-3-oxobutanoate (3f): Yield 78% (194.0 mg), Rf=0.40 (in EtOAc/Hexane: 1:4), light yellow crystal,(99.0% pure), Mp: 156~158°C. IR(KBr) υmax(cm–1): 3078 (aromatic C–H), 3039 (alkene=C–H), 2993 (–CH3), 1719 (C=O), 1680 (C=C). 1H NMR δ ppm 8.91 (s, 1H), 7.14 (m, 2H), 7.01 (m, 2H), 7.41 (m, 2H), 3.54 (s, 3H), 2.59 (s, 3H). 13C NMR (CDCl3) δ ppm 167.8, 162.3, 159.9, 146.3, 144.5, 129.2, 115.2, 102.0, 51.2, 18.7. MF=C12H11O3F.
Methyl 2-(4-nitrobenzylidene)-3-oxobutanoate (3g): Yield 76% (230.0 mg), Rf=0.25 (in EtOAc/Hexane: 1:4), yellow crystal, (99.0% pure), Mp: 125∼127°C. IR(KBr) υmax(cm–1): 3104 (aromatic C–H), 3047 (alkene=C–H), 2954 (–CH3), 1743 (C=O), 1657 (C=C). 1H NMR δ ppm 8.21 (m, 2H), 7.69 (s, 1H), 7.57 (m, 2H), 3.82 (s, 3H), 2.44 (s, 3H). 13C NMR (CDCl3) δ ppm 193.8, 187.2, 148.4, 139.0, 137.8, 130.2, 129.8, 123.9, 52.8 and 26.8. MF=C12H11O5N.
We have synthesized seven compounds on the NH4OAc catalyzed condensation of the active methylene compounds with the aromatic and heterocyclic aldehydes (Scheme 1). The structure of the products, substrate, yield of % are shown in Table 2.
| Entry | Aldehyde | Product | Watt/Time (second) | Yield (%) |
|---|---|---|---|---|
| 1a | 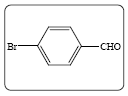 |
 |
300/60 | 87 |
| 1b | 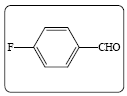 |
 |
300/60 | 82 |
| 1c | 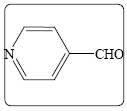 |
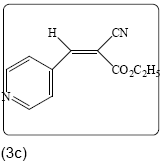 |
450/120 | 96 |
| 1d | 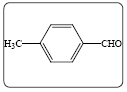 |
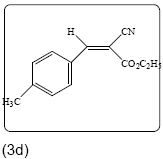 |
450/60 | 96 |
| 1e | 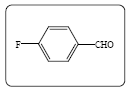 |
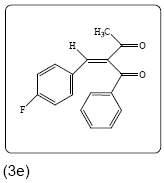 |
600/180 | 95 |
| 1f | 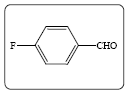 |
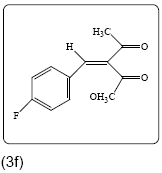 |
800/120 | 78 |
| 1g | 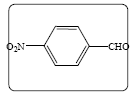 |
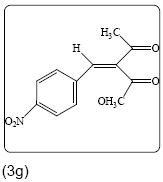 |
450/180 | 76 |
Table 2: Structure of the aldehydes, products, % yield and melting points of the microwave assisted Knoevengel condensation reaction of various aromatic aldehydes and active methylene compounds.
The reaction of p-bromobenzaldehyde with ethyl cyanoacetate in presence of catalytic amount of ammonium acetate under microwave irradiation resulted in the formation of ethyl-2-cyano-3-(4- bromophenyl) acrylate (3a) in 87% yield under solvent free condition. In a similar manner, a wide range of aldehydes including aromatic and heterocyclic reacts efficiently with the active methylene compounds 2 under same reaction condition to give the arylidene derivatives with yield% of 96 to 76. The structures are established based on FT-IR, 1H NMR, 13C NMR and MS spectroscopic analyses shown in Table 2.
The results representing antimicrobial activity of the synthesized compounds presented in Table 1. The activity of three typical independent compounds such as 3b, 3c and 3d are shown in Figure 1. From the antimicrobial activity test results, it has been observed that almost all the compounds except 3g showed the moderate antibacterial activity against the used pathogens. The highest activity 6.0 mm of zone of inhibition was found to have against K. oxytoca for the compounds 3b, 3c, 3d and 3f and the lowest activity 5.0 mm was found to be for the compound 3e. The compounds 3a and 3g were found to be inactive against the pathogen K. oxytoca . On the contrary, four compounds, out of seven, such as 3a, 3c, 3d and 3f were found to be active against the pathogen E. coli whereas compounds 3b, 3e and 3g were inactive against E. coli . The highest activity 8.0 mm of zone of inhibition was observed for the compound 3d and the lowest activity 7.0 mm of zone of inhibition was observed for the compound 3c. The compound 3g was found to be inactive in both the used pathogens. This might be due to the structure-activity relationship of the compound 3g, since, it contains two electron-donating groups (CH3O-, -CH3) and one strong electron-withdrawing group (-NO2). These differences in electronic properties might produce difference in antibacterial susceptibility and are inactive against Gram-negative pathogens (K. oxytoca and E. coli are both Gram-negative) [17]. However, the synthesized compounds are recommended as antibacterial agents.
In conclusion, we have synthesized seven arylidene derivatives by microwave irradiation using NH4OAc as catalyst under solvent free condition. This method could be applied to a wide range of aldehydes including aromatic and heterocyclic substrates. The advantages of this method are high yields, relatively short reaction times and low cost, simple experimental and isolation procedures. Six compounds, out of seven, were found to be moderate active against Gram-negative pathogenic bacteria. Finally, it is in agreement with the green chemistry protocols.
The authors are grateful to Professor Akiya Ogawa, Department of Applied Chemistry, Faculty of Engineering, Osaka Prefecture University, Japan for giving the opportunity in taking the 1H and 13C NMR spectrum. We gratefully acknowledged the University Research Centre, SUST for financial support.