Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2023)Volume 14, Issue 1
Background: HPLC-PDA method was developed using factorial design approach and validated for the estimation of hesperidin in orange peel powder. A 32 factorial design was applied to evaluate the effect of the two factors that are mobile phase composition and flow rate on the various chromatographic responses such as retention time, area, and tailing factor. Chromatographic separation was achieved using Sunniest ECO (250 mm × 4.6 mm, 5 μm) column as a stationary phase and optimized mobile phase consisted of Methanol: water in ratio of 51.5:48.5 v/v at a flow rate of 1.1 mL/min. Detection wavelength was 285 nm. The retention time of hesperidin was found to be 4.01 min.
Results: In validation studies, calibration curve was found to be linear over the range of 0.2-6 μg/mL with the regression coefficient of 0.996. The LOD and LOQ were found to be 0.066 μg/mL and 0.2 μg/mL; respectively. The % recovery of hesperidin was found to be between 96%-105%. The method was found to be precise and robust as the values of % RSD were found to be less than 2.0% for both intraday and interday precision study and in robustness study for hesperidin. The validated method was successfully applied for quantitative determination of hesperidin in dried orange peel powder.
Conclusion: Factorial design assisted HPLC-PDA method was developed and validated for the quantification of hesperidin in orange peel. This method can be used for routine quality control of orange peel powder using hesperidin as marker compound by the herbal manufacturer.
Hesperidin; Factorial design; Orange peel; HPLC-PDA
Hesperidin is flavonoids having health benefit potential [1]. Hesperidin (C28H34O15) is a flavanone glycoside comprised of the flavanone hesperetin and the disaccharide rutinose (Figure 1). It possesses anti-inflammatory, antioxidant, neuroprotective, anti-allergic, antihypertensive, and anti-arthritic activities [2-8]. It is also believed to be effective in cardiovascular diseases. Hesperidine is abundantly found in Citrus species [9].
Figure 1: Structure of Hesperidin.
As per ICH Q8 (R2), Quality by Design (QbD) is involves predetermined objectives and product/process understanding and control based on quality risk management [10]. One Factor at a Time (OFAT) approach for method development studies effect of variation in one factor at a time on response Quality by Design (QbD) which employs Design of Experiments (DoE) as significant concept. DoE approach is a methodical, scientifically analysed better understandable approach [11,12].
A UV spectroscopic method, few HPTLC methods, many HPLC methods, a UPLC method and a stability indicating HPLC method have been reported for estimation of hesperidin [13-29]. As per the literature review, no analytical method has been optimized using DoE for quantification of hesperidin in orange peel. Thus, the aim of present study was to develop and optimize HPLC method using 32 factorial design.
Chemicals and reagents
Reference standard of hesperidin (92% w/w) was purchased from Yucca Enterprises, Mumbai, India. Orange peels were procured from the local market of Silvassa, India. HPLC grade methanol and water were purchased from Fisher Scientific India Pvt. Ltd., Mumbai, India.
Instrumentation
The HPLC-PDA method was optimized using an HPLC chromatograph (Jasco, Japan) made up of a quaternary pump (PU-4180), sample introduction system (AS-4050) and photodiode array detector (MD-4017). ChromNAV software (version 2.0) was used for assessment of all data.
Preparation of standard stock solution
To make a standard stock solution of hesperidin, 2 mg of hesperidin was dissolved in 10 mL of volumetric flask containing 5 mL of methanol and was sonicated for 10 min. The final volume of the solution was made up to 10 mL with the methanol to achieve a stock solution containing 200 µμg/ml of hesperidin. The solution was filtered through a 0.2 μ sample filter.
Preparation of sample solution
Accurately weighed 5 mg of orange peel powder was taken into 50 mL of volumetric flask containing 25 mL of methanol and the solution was filtered through 0.2 μ sample filter sonicated for 10 min and then the volume was made upto 50 mL with methanol.
Initial method development
Initial method was developed by One Factor at a Time Approach (OFAT). Various mobile phases like methanol: water (95:5, v/v), methanol: water (75:25, v/v), methanol: water (65:35, v/v), methanol: water (45:55, v/v), methanol: water (50:50, v/v), methanol: water (25:75, v/v), methanol: water(40:60, v/v), etc. at a flow rate of 1 mL/min were tried and chromatographic responses like retention time, area, number of theoretical plates and tailing factor were evaluated but none of them produced the desired results. The mobile phase consisting of methanol: water (55:45, v/v) at a flow rate of 1 mL/min produced desired results which was further optimization by the factorial design.
Software aided method optimization
To optimize the chromatographic variables for hesperidin, 32 factorial experimental designs were used. The current work and it included three levels (low (-1), medium (0), and high (+1)) and two independent variables (A: mobile phase composition, B: flow rate) therefore, optimization only needed nine runs. Retention time (Y1), area (Y2), and tailing factor (Y3) where the chromatographic responses that were recorded during the trials. Using Design Expert (Version 11.0, Trial version), a 32 complete factorial design was acceptable for generating the response surface and producing various models. Analysis of Variance (ANOVA) was applied to the collected responses in order to determine the model's significance. 3D response surfaces plots, perturbation plots and predicted Vs actual plots were created to visualize the impact of the independent variable and their interactions on the responses. Each response's relevance to the optimization plan was demonstrated by the generation of regression equations.
Model optimization
The final mobile phase ratio and flow rate were optimized for the determination of hesperidin using Design Expert (Version 11) software after interpretation of various response surface plots and perturbation plots.
Model validation
All the trials using different mobile phase composition as stated in the above Table 1 were carried out. The effect of the solvents on chromatographic responses such as retention time, flow rate, and tailing factor. The results obtained were analyzed using Design Expert Software (DOE). From the optimized mobile phase, best mobile phase in the composition was used for model validation. The predicted responses and the results obtained were compared.
| Independent variable | |||
|---|---|---|---|
| Level used | |||
| Factor | Low (-1) | Medium (0) | High (+1) |
| A=Mobile phase (v/v) (Methanol: Water) | 45:55:00 | 50:50:00 | 55:45:00 |
| B=Flow (mL/min) | 0.5 | 1 | 1.5 |
| Dependent variable (Response) | |||
| Chromatographic response | Value | ||
| Y1 = Retention time (min) | 2.56 ≤ Y1 ≥ 14.4 | ||
| Y2=Area (mAU) | 1646163 ≤ Y2 ≥ 6765547 | ||
| Y3=Tailing Factor | 0.80 ≤ Y3 ≥ 1.076 | ||
Table 1: Independent and dependent variables selected for the 32 factorial designs.
| Run | Level | Factor | Response | |||
|---|---|---|---|---|---|---|
| Mobile phase composition (v/v) (A) | Flow rate (mL/min)(B) | Rt (min)(Y1) | Peak area (mAU)(Y2) | Symmetry factor (Y3) | ||
| 1 | -1, 0 | 45:55:00 | 1 | 6.77 | 3043588 | 1.0063 |
| 2 | -1, +1 | 45:55:00 | 1.5 | 4.7 | 1646163 | 1.074 |
| 3 | -1, -1 | 45:55:00 | 0.5 | 14.4 | 5602603 | 0.92 |
| 4 | 0, 0 | 50:50:00 | 1 | 5.05 | 2923739 | 0.998 |
| 5 | 0, +1 | 50:50:00 | 1.5 | 3.27 | 1836632 | 1.065 |
| 6 | 0, -1 | 50:50:00 | 0.5 | 10.1 | 6765547 | 0.805 |
| 7 | +1, 0 | 55:45:00 | 1 | 4.01 | 2937779 | 0.948 |
| 8 | +1, +1 | 55:45:00 | 1.5 | 2.56 | 1831801 | 1.076 |
| 9 | +1, -1 | 55:45:00 | 0.5 | 8.06 | 6593448 | 0.8 |
Table 2: Observed response for 9 runs for hesperidin.
| Response | Model | R2 | Adjusted R2 | Predicted R2 | SD | % CV | Adequate Precision |
|---|---|---|---|---|---|---|---|
| Retention time (Y1) | Quadratic | 0.9914 | 0.9853 | 0.9159 | 0.3856 | 6.34 | 42.3326 |
| Area (Y2) | Quadratic | 0.9886 | 0.9805 | 0.897 | 2.415E+05 | 7 | 30.1831 |
| Tailing factor (Y3) | Quadratic | 0.9707 | 0.9498 | 0.7746 | 0.0198 | 2.03 | 21.4231 |
Table 3: Summary of statistical analysis for various dependent variables (chromatographic responses).
| Response | Regression equations |
|---|---|
| Retention time | Y1=+4.31-1.27*A-6.37*B+0.88*A2+0.79*B2 |
| Area | Y2=+5.51-1.72*A-1.81*B+1.34*A2+0.62*B2 |
| Tailing factor | Y1=+0.96-0.12*A-0.057*B+0.038*A2+0.079*B2 |
Table 4: Regression Equation for various dependent variables (chromatographic responses).
Final chromatographic conditions
Separation was achieved on Sunniest ECO C18 column (250 mm × 4.6 mm, 5 μm). Elution was carried out in isocratic mode using mobile phase, methanol: water (51.5: 48.5 v/v) at flow rate of 1.1 mL/min. Injection volume was kept at 20 μL. Detection was carried out at 285 nm and run time was 10 min. The mobile phase was filtered before use through a 0.45 μ membrane filter (Sartorius Stedium Biotech, Germany) and sonicated for 10 min.
Method validation
The developed analytical method was validated according to the ICH guidelines for various parameters such as system suitability, specificity, LOD, LOQ, linearity and range, accuracy, precision and robustness.
Specificity
Specificity was evaluated by injecting blank, standard solution of hesperidin and sample solutions of orange peel. Any interference from the blank and sample components with the peak of hesperidin was checked..
System suitability
System suitability was evaluated by injecting six injections of standard solutions of hesperidin (6 μg/mL) to HPLC under optimized chromatographic conditions. The various parameters were evaluated such as the number of theoretical plates, tailing factor and repeatability of peak area.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The S/N ratio approach was used to determine the LOD and LOQ. The S/N ratio was calculated by estimating noise in the range of 5 to 7 min after a lower concentration of hesperidin was administered. Concentrations that resulted in an S/N ratio of 3 and 10 were considered as LOD and LOQ, respectively.
Linearity and range
The linearity was tested by injecting the six different concentrations of hesperidin standard solutions ranging from 0.2 to 6 µμg/mL. Six different concentrations were generated from a 100 μg/mL standard stock solution by pipetting out 0.02, 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 into separate 10 mL volumetric flasks, and the final volume was made up to 10 mL with methanol to get the concentration between 0.2 μg/mL to 6 μg/mL. The resultant solutions were injected into an HPLC system in triplicates under optimum chromatographic conditions, and the area was calculated. For each concentration, the average area was computed. The calibration curve was drawn, and the R2 for hesperidin was computed.
Accuracy
The accuracy was carried out at three different concentration levels (80%, 100%, 120%). Accurately weighed 5 mg of orange peel powder was dissolved in 10 mL of methanol and then the solution was sonicated for 10 min and filtered. Accurately measured 1 mL of this solution was spiked with standards hesperidin solution having concentration of 3.2 μg/mL, 4.0 μg/mL, and 4.8 μg/mL. These solutions were injected in triplicates into HPLC. Also, standard hesperidin solutions (3.2 μg/mL, 4.0 μg/mL, and 4.8 μg/mL) and spiked orange peel sample were also separately injected in triplicate. The percentage recovery at each level was determined by subtracting sample values from spiked values and comparing them with standard values.
Precision
The proposed method's precision was assessed by looking at the intraday and interday precision of sample solutions at three different concentration levels (80%, 100% and 120%). In triplicates, sample solutions of orange peel powder containing hesperidin at three concentration levels (4.0 mg/50 mL, 5.0 mg/50 mL and 6.0 mg/ 50 mL) were injected into HPLC. The peak area of hesperidin was measured at each concentration level, and the % RSD was computed. The intraday and interday precision experiments were carried out in the same way. In the intraday precision study, the analysis was performed twice on the same day at various time intervals. The analysis for the interday precision was performed over two days.
Robustness
The robustness for the devised technique was evaluated by altering the method parameters such as flow rate (1.2 and 1.0 mL/min), mobile phase composition (Methanol: Water (52:48, v/v) and 51:49, v/v) and wavelength (283 nm and 287 nm). One parameter was altered at a time. Hesperidin standard solution (4.0 μg/mL) was injected six times, followed by a sample solution (hesperidin 5 mg/50 mL). For both changed and unchanged conditions, % RSD of area acquired from six injections of standard solutions and % w/w of hesperidin were calculated.
Analysis of orange peel powder for content of hesperidin
Standard solution containing hesperidin (4.0 μg/mL) and sample solution was injected in triplicate, average area was noted and % w/w of hesperidin was calculated.
Initial method development
The absorbance maximum of hesperidin was found to be 285 nm hence was selected as detection wavelength during the development of HPLC method for hesperidin.
Method development based on Design of Experiment (DOE)
A 32 full factorial design using 9 experimental runs was carried out for the optimization of proposed HPLC method. The independent variables, levels and dependent variables selected for the DOE are given in Table 1. Mobile phase composition and flow rate were selected as independent variables as they were key factors to change chromatographic behaviour of hesperidin.
The results of 9 experimental runs carried out and observed responses are given in Table 2. The difference between predicted R2 and adjusted R2 was not more than 0.2 for all responses demonstrating that there was a reasonable agreement between them. Adequate precision greater than 4.0 indicated that there was adequate signal for all responses. The best fitted model for retention time, area and tailing factor was found to be quadratic model (Table 3). The regression equations for various chromatographic responses obtained from ANOVA analysis are also presented in Table 4. A positive value in the equation represented the factor that favours the optimization, while a negative value indicated an inverse relationship between the independent variable and the response. It is clear from the equations that variable A (mobile phase) and variable B (flow rate) had a negative effect on all responses. The square of variable A (A2) and variable B (B2) had a positive effect on all responses.
The Sum of Squares (SS) in ANOVA indicated that the contribution of variable B (SS 80.89) was more on retention time as compared to variable A (SS 21.06), the contribution of variable B (SS 3.104 × 1013) was more on area as compared to variable A (SS 1.911 × 1011), and the contribution of variable A (SS 0.0050) was less on tailing factor as compared to variable B (SS 0.0787). For each chromatographic response, all the model terms were found to be significant. The calculated F-value for the models of retention time, area, and tailing factor were found to be 162.10, 121.37 and 46.41; respectively. 3D surface plots were obtained to determine the relationship between the variables and various chromatographic responses. The surface plots also confirmed negative effect of mobile phase composition and flow rate on all responses. As the proportion of methanol in mobile phase composition and flow rate was increased retention time, area and tailing factor were found to be decreased. As per perturbation plots, the line B was found to be more steeper than line A indicating that mobile phase flow rate had more effect on all responses than the mobile phase composition. Based on predicted v/s actual plots, it was observed that there was sufficient agreement between real data and predicted data which were achieved from the models (Figure 2).
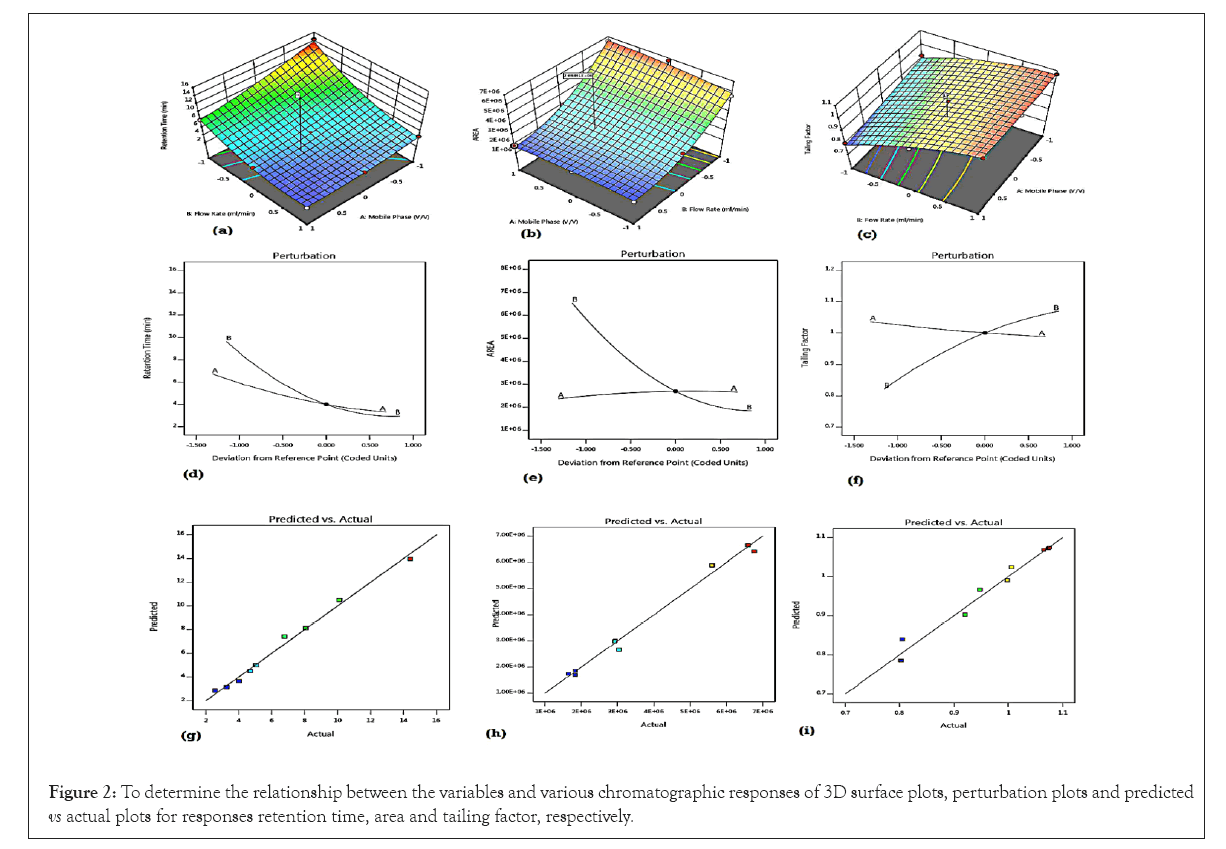
Figure 2: To determine the relationship between the variables and various chromatographic responses of 3D surface plots, perturbation plots and predicted vs actual plots for responses retention time, area and tailing factor, respectively.
Model validation
From the optimization plot, the best mobile phase combination was methanol: water in the ratio of 51.5: 48.5, v/v and flow rate was 1.1 ml/min respectively. The predicted responses for this mobile phase were retention time of hesperidin at 4.00 min with desirability 1.0 which was given by the software. In order to validate the model, the trial was conducted with the optimum mobile phase and flow rate obtained. The results obtained from the trials were found to be retention time 4.207 min. Thus, the results obtained from the trials were found to be comparable with the prediction. Hence the model was successfully validated.
Method validation
Specificity: The proposed HPLC method was found to be specific as there was no interference found from the solvent, mobile phase or any other components present in orange peel powder (Figure 3).
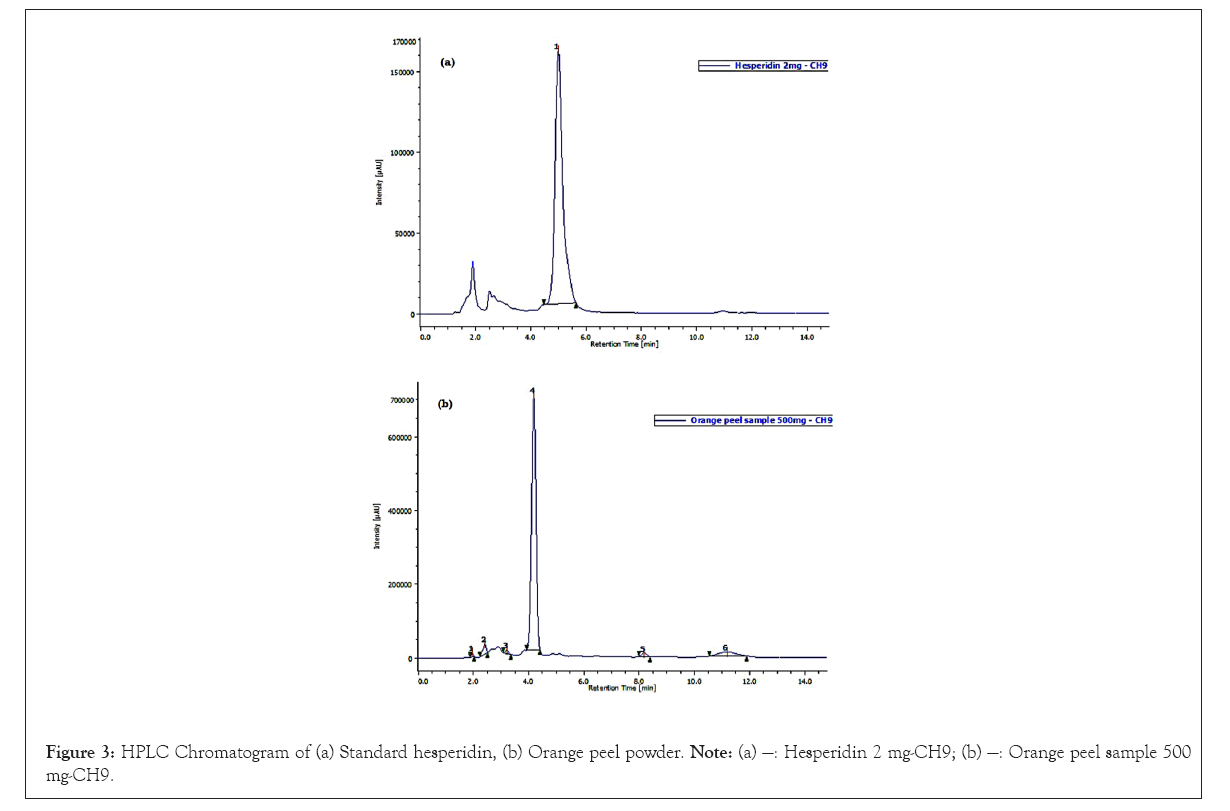
Figure 3: HPLC Chromatogram of (a) Standard hesperidin, (b) Orange peel powder. Note: (a) —: Hesperidin 2 mg-CH9; (b) —: Orange peel sample 500 mg-CH9.
System suitability: The number of theoretical plates which is a measure of column efficiency was found to be more than 3000. The tailing factor for the peak of hesperidin was found to be 1.05. The repeatability of peak area was found to be less than 2.0. As the results of system suitability study were within the standard specifications, the optimized method was found to be suitable for the analysis of hesperidin.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOQ of the proposed HPLC method for hesperidin were found to be 0.066 μg/mL and 0.2 μg/mL; respectively. The developed method was found to be sensitive as it could detect and quantitate microgram levels of hesperidin.
Linearity: The proposed method showed linearity over concentration range of 0.2-6 μg/mL for hesperidin with correlation coefficient 0.9933 demonstrating and acceptable data fit to the regression line Y=6941.9X+15194.
Accuracy: The % recovery of hesperidin was found in the range between 96%-105% which is within the acceptance limit (Table 5). As the % recovery for hesperidin was near to 100, the proposed method was found to be accurate.
| % Level | Sample conc (mg/100 mL) | Standard conc(µg/ml) | Mean % recovery ± SD |
|---|---|---|---|
| 80 | 5 | 3.2 | 96.0000 ± 2.0 |
| 100 | 5 | 4 | 101.0000 ± 1.0 |
| 120 | 5 | 4.8 | 105.3333 ± 1.5 |
Table 5: Accuracy of the proposed HPLC method for hesperidin.
Precision: Intraday as well as interday precision were carried out for evaluating the precision of the proposed method and the % RSD was found to be less than 2 at all concentration levels for both intraday and interday precision studies (Table 6). Thus, the developed method was found to be precise.
| Concentration (mg 50 mL) | Intraday precision(Mean area ± SD) | Interday precision(Mean area ± SD) | ||
|---|---|---|---|---|
| Day 1 | Day 1 | Day 2 | Day 3 | |
| 4 | 25324.33 ± 1.5 | 25494.33 ± 1.5 | 23514.67 ± 2.5 | 24594.67 ± 2.5 |
| 5 | 32241.67 ± 2 | 31995.33 ± 2.0 | 34234.6 ± 1.5 | 35102.33 ± 1.5 |
| 6 | 48176.33 ± 1.0 | 49564.67 ± 1.0 | 49261.33 ± 2.5 | 48774.33 ± 2.5 |
Table 6: Results of intra-day and inter-day precision studies of proposed HPLC.
Robustness: In robustness study % RSD was found to be less than 2.0% in case of area of standard solutions and % w/w of hesperidin was found to be between 90%-110% for both altered and unaltered conditions. The results of robustness study for both altered and unaltered conditions are shown in Table 7.
| Parameter | Retention time (min) | Area | %RSD | %w/w content of hesperidin |
|---|---|---|---|---|
| Flow rate (ml/min) | ||||
| 1 | 4.615 | 46854 | 0.0524 | 3.70 % w/w |
| 1.2 | 3.812 | 44282 | 0.0962 | 3.59 % w/w |
| Detection wavelength(nm) | ||||
| 283 | 4.187 | 44063 | 0.053 | 3.72 % w/w |
| 287 | 4.185 | 42379 | 0.1277 | 3.69 % w/w |
| Mobile phase (Methanol: Water, v/v) | ||||
| (48: 52, v/v) | 4.075 | 46675 | 1.865463 | 3.64 % w/w |
| (49: 51, v/v) | 4.301 | 46872 | 1.525296 | 3.74 % w/w |
Table 7: Results of robustness study for unaltered conditions for HPLC method.
Quantification of hesperidin in orange peel powder by HPLC: The orange peel powder was analyzed by the proposed HPLC method. The % w/w of hesperidin in orange peel powder was found to be 3.65% w/w.
Factorial design was employed for optimization of HPLC method for quantification of hesperidin in orange peel. Factorial design had helped in identification of best chromatographic conditions for desired responses. Interactions between mobile phase and flow rate were evaluated. Flow rate was major influencing variable than mobile phase composition in optimization. The method was validated for various parameters as per ICH guidelines and was used for quantification of hesperidin present in dry powder of orange peel. The proposed method was found to be sensitive, accurate, precise and robust.
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef]
Citation: Desai P, Thakor N, Desai S (2023) Factorial Design Based HPLC-PDA Method Optimization for Quantification of Hesperidin in Orange Peel. J Chromatogr Sep Tech.14:494
Received: 14-Dec-2022, Manuscript No. JCGST-22-20907; Editor assigned: 16-Dec-2022, Pre QC No. JCGST-22-20907 (PQ); Reviewed: 03-Jan-2023, QC No. JCGST-22-20907; Revised: 12-Jan-2023, Manuscript No. JCGST-22-20907 (R); Published: 25-Jan-2023 , DOI: 10.35248/2157-7064.23.14.494
Copyright: © 2023 Desai P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.