Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2023)Volume 8, Issue 1
Inflammatory Bowel Disease (IBD) in children is becoming more prevalent globally. According to epidemiology studies, it affects many children who live in poor sanitation and unhygienic environments. According to earlier research done on an animal model and in the human population, SCFAs have been linked to gut inflammation biomarkers. However, studies on the association of gut inflammation with SCFAs were scarce in Ethiopia. Thus, the purpose of this study was to assess the relationship between fecal SCFAs and gut inflammation in Hawassa City, Sidama Region, Ethiopia. A community-based cross-sectional study design was employed, and 82 children were included. A simple random sampling technique was used to select children from the source population. Independent samples-t-test, factorial ANOVA, and linear multiple regression were used to analyze data. The mean (± SD) of total SCFAs was 1.57 (± 0.16) μmoles/g stool sample. Gut inflammation was strongly and negatively correlated with total fecal SCFAs (r=-0.58, p<0.05). However, there was a moderate and positive correlation (r=0.38, p<0.05) between pH status and gut inflammation. Antibiotic exposure in the three months prior to the data collection was significantly associated with both the total and the individual SCFAs. Children who were exposed to any antibiotic in the three months prior to the data collection had significantly lower SCFAs concentration (1.51 ± 0.11, p=0.021) than their counterparts. Similarly, antibiotics-exposed children had significantly lower acetic acid (1.3 ± 0.12, p=0.032), butyric acid (0.79 ± 0.13, p=0.027), and propionic acid (0.86 ± 0.10, p=0.023) than their counterpart. Antibiotic exposure in the past three months prior to the data collection had a significant impact (F (1,75)=4.2, p=0.034) on the total SCFAs. About 9.3% variation in the total SCFAs was explained by antibiotic exposure. Likewise, a diet had a statistically significant effect (F (1,75)=3.6, p=0.041) on the total SCFAs. Diet explained about 8.6% of the total variation in the total SCFAs. Inflammation in the gut was substantially correlated with fecal SCFAs. Low dietary diversification and exposure to antibiotics both had an impact on the amount of fecal SCFAs. Government policy should concentrate on raising community awareness about proper antibiotic usage and enhancing the nutritional diversity of children under the age of five using the food options.
Association; Bacterial; Gut inflammation; Hawassa; SCFAs; Under five
The inflammation of an intestinal tract's mucosal lining is a sign of the disorder known as Inflammatory Bowel Disease (IBD). The two most common IBD subtypes are Crohn's Disease (CD) and Ulcerative Colitis (UC) [1]. Observational studies have shown that the disorder also shows signs of aberrant histopathology, permeability issues, and reduced nutrient absorption [2]. In many developed nations, it is estimated that more than 0.3 percent of the population suffers from IBD, and the prevalence is rising in emerging nations [3,4]. Inflammatory Bowel Disease (IBD) in children is becoming more prevalent globally. According to epidemiology studies, it affects many children who live in poor sanitation and unhygienic environments [5,6]. Broadly, the disorder is caused by a complex interplay between genetic, immunologic, microbial, and environmental factors [7,8]. Relatively, early nutrition and intestinal microbiota have a big impact on the pathology of gut inflammation [9,10]. Early-life malnutrition, enteric infections, and small intestinal bacterial overgrowth may encourage intestinal barrier disruption and the translocation of intestinal bacterial products, which in turn may result in low-grade, chronic, subclinical systemic inflammation [11].
The human body system is home to trillions of microorganisms that engage in a variety of biological interactions, including commensal, parasitic, and mutualistic. One of the body regions where commensal bacteria predominately live is the gastrointestinal tract [12,13]. It has been discovered that certain of these bacteria support gut health in several modes. Others have been reported to cause gut inflammation through improper activation of the intestinal epithelial immune system. Bacterial genera like Campylobacter, Shigella, Yersinia, and salmonella were identified as the cause of environmental enteropathy that was characterized by gut inflammation [14]. In individuals with inflammatory bowel illnesses, there is frequently an imbalance in the gut microbiome and a reduction in the number of bacteria that produce SCFAs (acetic, propionic, and butyric acid) [15].
Short-chain Fatty Acids (SCFAs) have received a lot of interest as signal molecules between the gut lumen and the brain among the microbial metabolites in the gut [16]. These metabolites are essential for gut health because they provide energy to enterocytes, regulate metabolism, and have anti-inflammatory and anti-oxidant characteristics [17]. Inhibiting LPS-induced cytokine synthesis in human T cell proliferation and cytokine production was another indication of SCFAs' strong immunoregulatory abilities [18]. The generation of SCFAs from the fiber by the Lactobacillus and Bifidobacterium genera has shown the efficacy of soluble fibers, such as oligosaccharides and resistant starch, in alleviating gastrointestinal inflammation. Thus, these acids could be employed as indicators of the health of those probiotic bacteria that are crucial for early childhood growth [18-20].
In animal studies, adding probiotics to the diet reduced levels of the pro-inflammatory cytokine’s interleukin-6 and interferongamma. The colon's production of SCFAs served as evidence for the mechanism [21,22]. According to earlier research done on an animal model and in the human population, SCFAs have been linked to gut inflammation biomarkers. However, studies on the association of gut inflammation with SCFAs were scarce in Ethiopia. As a result, the purpose of this study was to assess the relationship between fecal SCFAs and gut inflammation in Hawassa City, Sidama Region, Ethiopia.
Study area and period
This study was conducted at Hawassa, the capital city of the Southern Nation, Nationalities and Peoples Region (SNNPR), and Sidama National Regional State. The city is 270 km away from Addis Ababa, the capital city of Ethiopia. Hawassa is bordered by the Wondo Genet District in the East, the South Dore Bafeno District in the South, the Oromia Region in the North, and the Hawassa Lake and Oromia Region in the West. The metropolis is divided into 8 sub-cities and 32 kebeles. According to the document of the housing and population census, the projected population of the Hawassa metropolis administration in 2011 was 329,734 out of which 169,677 were males and 160,057 were females [23]. The study was carried out from September 2021 to February 2022.
Source population and study design
The source population for this study was all children aged 6-59 months and living in Hawassa City, Sidama Regional State. A community based cross-sectional study design was employed to gather the data.
Sample size determination
The sample size was determined using G* power by considering the statistical test of linear multiple regression, two-sided, effect size 0.13, α value of 0.05, and β-value of 0.10. Thus, the total sample size was calculated to be 83. However, a sample size of 82 was used for the analysis.
Sampling technique
Primary sampling unit was the sub-city at Hawassa city, and then the households in the sub-city were the secondary sampling unit. In general, four sub-cities (Tabor, Meneharia, Tulla, and Haik Dar) based on the city’s demographic data in 2021 obtained from the health bureau. In each sub-city, having under-five children and a willingness to participate in the study were the criteria for including a household in the study. The tertiary sampling unit, a child-mother pair, was selected among the source population through a simple random sampling.
Inclusion and exclusion criteria for selecting children
Children with the following inclusion criteria were eligible for t his study: Age of 6 59 months, the beginning of solid food intake, and exclusively breastfed for under two children. Children who had travel history, had a chronic disease were excluded from this study.
Data collection tools and procedures
For anthropometric data collection from children, permission was obtained from the parents. Data were collected from children aged 24-59 months by measuring their weight in underwear and without shoes with an electronic scale (Type SECA 861 or SECA 813, Hamburg, Germany) to the nearest 0.1 kg and their height in the Frankfort plane with a telescopic height instrument (Type SECA 225 or SECA 214) to the nearest 0.1 cm. Data from children aged 6-23 months, on the other hand, were collected by measuring the child's weight and that of the mother/caregiver, then subtracting the weight of the mother/ caregiver from the sum weight of the child and the mother/ caregiver. The height was measured using an accurately graduated length board and recorded to the nearest millimeter. The age of the children was obtained from a parental recall using an events calendar. The height and the weight of the children were measured twice, and the average was taken. The measuring instruments were calibrated at least twice a day in each case. According to the World Health Organization (WHO, 2008), wasting, stunting, and being underweight are defined as Z-scores of less than -2 standard deviations of weight for height, height for age, and weight for age, respectively [24]. An interviewer-administered questionnaire was used after a 5% sample pre-test. The questionnaire was translated into the Amharic language, the official language of Ethiopia, and the data collectors were fluent speakers of Amharic. The respondents were at least capable of speaking and listening to Amharic. The collected data was translated back to English by a proficient translator to ensure accuracy and consistency. The data collection tool for dietary diversity score was adapted from the world health organization’s guidelines proposed for assessing infant and young child feeding practices. Eating 4 or more of the 7 food groups means that the child is probably to consume at least one animal food source and at least one fruit or vegetable in addition to the staple food (grains, roots, or tubers) in the last 24 hours. Four food groups should be drawn from the list of seven food groups: Grains, roots and tubers, legumes and nuts; dairy products (milk yogurt, cheese); meat, fish, poultry, and liver/organic meat; eggs; vitamin A-rich fruits and vegetables; and other fruits and vegetables [25]. Data were gathered from the mother’s or caregiver’s recall of foods given to the child in the past 24-hours before the interview.
Besides, data on household sanitation and hygiene, a child’s mode of delivery, breastfeeding practice, and taking antibiotics were collected. Breastfeeding practice was assessed based on the beginning of complementary feeding at 6 months, continued breastfeeding for up to 2 years, colostrum feeding, and exclusively breastfeeding for 6 months. It was rated as “optimum” if all those conditions were fulfilled, and “suboptimal” if one or more of those were missing. Sanitation and hygiene status was assessed according to the [26]. For antibiotic exposure, the mother/caregiver was asked about the taking of any antibiotics by the child for the past three months before the survey.
Fecal SCFAs and pH test
Fecal SCFAs were measured by using UV/VIS spectroscopy (UV-1700, Shimadzu, Japan) and quartz cuvette having a 1cm path length as a sample holder under the wave length ranging from 200–400 nm. A characteristic spectrum of each SCFAs was obtained by scanning the acids of the analytical grade (99.8%). About 400 mg stool samples were diluted with 1 mL distilled water, homogenized by using a vortex, and centrifuged at 6300 rpm at 4°C for 15 minutes. From the supernatant, 0.75 mL of fecal water was transferred to a fresh tube and subjected to centrifugation at 20,000 × g at 4°C for 15 minutes. This step was repeated once with 600 μL of supernatant recovered from the previous centrifugation. Finally, about 560 μL of each sample solution was transferred to a test tube for further analysis [27]. A standard solution was prepared for the major colonic SCFAs: Acetic acid, butyric acid, and propionic acid (all the acids were of analytical grade, 99.8%). To construct a standard curve, the mixture of the acids was adjusted in the concentrations level of 20 mg/L, 15 mg/L, 10 mg/L, 5 mg/L and 1 mg/L. Then, using the total maximum absorbance, the concentration of all SCFAs was estimated. Additionally, the mathematical matrices were used to determine the concentration of each SCFA [28]. The stool was appropriately blended and homogenized with 10 ml of distilled water before to the fecal pH test. Complete immersion of the pH meter's probes in the mixture for 1 minute was followed by recording of the reading. The test was conducted with the help of the portable pH meter (Hanna Instruments, USA) [29].
Gut inflammation test
This was tested through fecal leukocytes count. Fresh stool samples were examined for the presence of fecal leukocytes on smears made onto glass slides within 20-30 minutes after the stools were collected as described in clinical diagnosis guidelines [30,31]. The stools for microscopic examination were chosen from an area with blood or mucus if present. Each sample was stained with methylene blue (Himedia, Mumbai, India) and examined by an experienced laboratory technician who was blinded to the source of the sample (stunted or not stunted children). Microscopic examination of the preparations was done by examining each for 10 minutes using an optical light microscope. The numbers of Leukocytes per field (Lpf) (Oil immersion field, magnification, 1000x) were determined in at least 20 fields. The average results were categorized as follows: 3 to 5 Lpf, 6 to 10 Lpf, 11 to 15 Lpf, or 16 Lpf. Based on previous studies, we chose a cutoff point of 10 Lpf to decide the presence of a gut inflammation related to an enteric infectious bacteria [32].
Bacterial culture as a conformation test
Stool samples were collected using clean, dry, and leak-proof stool cups and immediately placed into the Cary-Blair transport medium (Oxoid Ltd., Basingstoke, UK). Samples were transported to Hawassa University’s Food Microbiology Laboratory in cold boxes with ice packs within 2 hours of collection for further processing. Stool samples were directly inoculated onto MacConkey agar, Salmonella-Shigella agar, and Xylose Lysine Deoxycholate agar after enrichment with Selenite cystine broth and incubated at 37°C for 18-24 hours. After incubation, bacterial isolates were identified to the genus level by the colony morphology and biochemical characteristics of the isolates [33].
Data analysis
Before the data processing, each filled questionnaire was checked for completeness and consistency. Then, the data were coded, entered and cleaned using SPSS version 20⋅0 (SPSS, Inc., Chicago, IL, USA). Eventually, the data were analyzed by using linear multiple regression, independent samples t-test, and factorial ANOVA.
Socio-demographic and clinical characteristics: Children and mothers
The majority of the children (54.9%) were under the age 13-35 months. Relatively, more male (54%) than female children were included in this study. About half (47.5%) of the children were recruited from Haik Dar sub-city. Nearly, one quarter (24%) of the children were born through either obligatory or selective cesarean section. Almost all (93.7%) of the children completed the full package of vaccination. Above one third (36.6%) of the children had gut inflammation raging form low to high level. Among the children having gut inflammation, about 41.5% were stunted. Even if more than half (57%) of the children began solid food intake at the recommended time (6th month), cloth to half (42.7%) of children began solid food intake at 4th month and earlier. Among the collected stool samples, more than half (54%) had yellow color, and the majority (73.2%) of the stool samples were less acidic (5-6.8 pH) by adjusting the surrounding temperature to 25°C. With regard to the mothers’ characteristics, the majority (67%) were merchants, and about 67.8% completed primary school and below (Table 1).
| Characteristics | Category | Frequency | Percentage |
|---|---|---|---|
| Stool color | Black | 24 | 29 |
| Brown | 14 | 17 | |
| Yellow | 44 | 54 | |
| Stool pH | More acidic | 4 | 4.9 |
| Moderate acidic | 18 | 21.9 | |
| Less acidic | 60 | 73.2 | |
| Residence | Haik Dar | 39 | 47.5 |
| Meneharia | 17 | 20.7 | |
| Tabor | 10 | 12.3 | |
| Tulla | 16 | 19.5 | |
| Sex | Male | 44 | 54 |
| Female | 38 | 46 | |
| Age in month | 06-Dec | 10 | 12.2 |
| 13-35 | 45 | 54.9 | |
| 36-59 | 27 | 32.9 | |
| Gut inflammation | Inflamed | 30 | 36.6 |
| Non inflamed | 52 | 63.4 | |
| Nutritional status | Stunted | 34 | 41.5 |
| Not stunted | 48 | 58.5 | |
| Mode of delivery | Cesarean section | 24 | 29.3 |
| Vaginal | 58 | 70.7 | |
| Immunization status | Complete | 77 | 93.9 |
| Not complete | 5 | 6.1 | |
| Time to begin solid food intake in months | 3 | 9 | 11 |
| 4 | 26 | 31.7 | |
| 6 | 47 | 57.3 | |
| Maternal education | Illiterate | 33 | 40.2 |
| Grade 1-8 | 30 | 36.6 | |
| Secondary school | 14 | 17 | |
| College and above | 5 | 6.2 | |
| Maternal occupation | Merchant | 55 | 67 |
| Homemaker | 15 | 18.3 | |
| Government employee | 12 | 14.7 |
Table 1: Socio-demographic and clinical characteristics of the children and mothers at Hawassa City, 2022.
The analytical grade (99.8%) of the three major SCFAs had peaks at 220 (nm), 240 (nm) and 260 (nm) for butyric, propionic and acetic acids respectively (Figures 1 and 2).
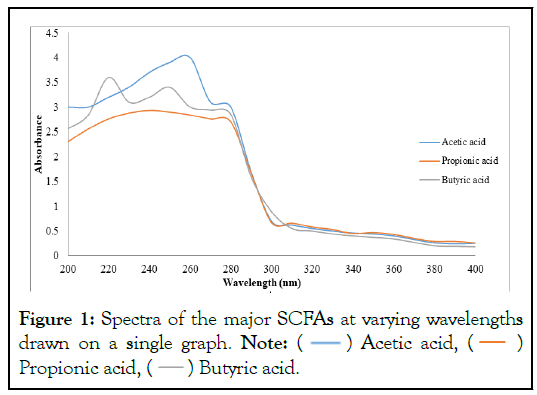
Figure 1: Spectra of the major SCFAs at varying wavelengths
drawn on a single graph. Note:  Acetic acid,
Acetic acid,  Propionic acid,
Propionic acid,  Butyric acid.
Butyric acid.
Figure 2: Standard curves of the three SCFAs, A- acetic acid, B-butyric acid, and C- propionic acid: Absorbance against concentration at five levels.
The mean (± SD) of total SCFAs was 1.57 (± 0.16) μmoles/g stool sample. Gut inflammation was strongly and negatively correlated with total fecal SCFAs (r=-0.58, p<0.05). However, there was a moderate and positive correlation (r=0.38, p<0.05) between stool pH status and the gut inflammation (Figure 3).
Figure 3: A negative correlation between total SCFAs and gut inflammation and a positive correlation between pH and gut inflammation.
Antibiotic exposure in the three months prior to the data collection was significantly associated with both the total and the individual SCFAs. Children who exposed to any antibiotic in the three months prior to the data collection had significantly lower SCFAs concentration (1.51 ± 0.11, p=0.021), than their counter part (Table 2). Similarly, antibiotics exposed children had significantly lower acetic acid (1.3 ± 0.12, p=0.032), butyric acid (0.79 ± 0.13, p=0.027), and propionic acid (0.86 ± 0.10, p=0.023) than their counter part (Table 2).
| Variables | Category | TSCFAs(µ, SD) | Acetic acid | Butyric acid | Propionic acid |
|---|---|---|---|---|---|
| Breastfeeding status | Optimum(n=56) | (1.57 ± 0.16) | (1.37 ± 0.15) | (0.83 ± 0.12) | (0.90 ± 0.18) |
| Sub-optimum (n=26) | (1.59 ± 0.14) | (1.35 ± 0.13) | (0.84 ± 0.16) | (0.93 ± 0.14) | |
| Mode of delivery | Vaginal (n=58) | (1.57 ± 0.16) | (1.36 ± 0.16) | (0.84 ± 0.11) | (0.92 ± 0.12) |
| CS (n=24) | (1.57 ± 0.13) | (1.35 ± 0.14) | (0.82 ± 0.15) | (0.91 ± 0.11) | |
| Age_cate in months | 6-12(n=10) | (1.54 ± 0.22) | (1.3 ± 0.2) | (0.79 ± 0.2) | (0.88 ± 0.18) |
| 13-35(n=45) | (1.59 ± 0. 15) | (1.4 ± 0.15) | (0.85 ± 0.15) | (0.93 ± 0.15) | |
| 36-59(n=27) | (1.55 ± 0.12) | (1.36 ± 0.12) | (0.80 ± 0.13) | (0.90 ± 0.11) | |
| Immunization | Complete(n=77) | (1.57 ± 0.15) | (1.4 ± 0.11) | (0.83 ± 0.15) | (0.91 ± 0.16) |
| Not complete(n=5) | (1.57 ± 0.15) | 1.34 ± 0.16) | (0.82 ± 14) | (0.93 ± 0.17) | |
| Sex | Male(n=44) | (1.58 ± 0.15) | (1.37 ± 0.14) | (0.84 ± 0.15) | (0.91 ± 0.15) |
| Female(n=38) | (1.57 ± 0.16) | (1.35 ± 0.15) | (0.82 ± 0.13) | (0.91 ± 0.14) | |
| Antibiotic exposure | Exposed(n=30) | (1.51 ± 0.11) | (1.3 ± 0.12) | (0.79 ± 0.13) | (0.86 ± 0.10) |
| Not exposed(n=52) | (1.62 ± 0.17)* | (1.45 ±0.18)* | (0.85 ± 0.15)* | (0.94 ± 0.16)* | |
| Nutritional status | Stunted(n=34) | (1.52 ± 0.14) | 1.4 ± 0.17) | (0.81 ± 0.11) | (0.87 ± 0.15) |
| Not stunted(n=48) | (1.62 ± 0.16) | (1.3 ± 0.15) | (0.84 ± 0.16) | (0.93 ± 0.16) |
Note: - μ- mean; SD-standard deviation; * -significant at p<0.05; TSCFAs- Total Short-chain Fatty Acids
Table 2: Log-transformed mean difference of SCFAs among under five children grouped under different categories (μmoles/g stool).
To compare the mean difference of total SCFAs among the under-five children as a function of various categorical independent variables, we did a factorial ANOVA analysis at the p-value of <0.05. Based on the output of the analysis, antibiotic exposure in the past three months prior to the data collection had a significant impact (F (1.75)=4.2, p=0.034) on the total SCFAs. About 9.3% variation in the total SCFAs was explained by the antibiotic exposure. Likewise, diet had statistically significant effect (F (1.75)=3.6, p=0.041) on the total SCFAs. Diet explained about 8.6% of the total variation in the total SCFAs. However, the remaining variables, the households’ sanitation (F (1.75)=2.2, p=0.14), households’ hygiene (F (1,75)=0.25, p=0.6), and age of the children (F (2.75)=1.2, p=0.3) had not significantly affected the variation (Table 3).
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. | Effect size |
|---|---|---|---|---|---|---|
| Corrected Model | 0.270a | 6 | 0.045 | 1.986 | 0.078 | 0.187 |
| Intercept | 49.753 | 1 | 49.753 | 2191.9 | 0 | 0.967 |
| Antibiotics Expo | 0.095 | 1 | 0.095 | 4.202 | 0.034 | 0.093 |
| Diet | 0.082 | 1 | 0.082 | 3.604 | 0.041 | 0.086 |
| Sanitation | 0.049 | 1 | 0.049 | 2.175 | 0.144 | 0.028 |
| Hygiene | 0.006 | 1 | 0.006 | 0.247 | 0.62 | 0.003 |
| Age_cate | 0.054 | 2 | 0.027 | 1.179 | 0.313 | 0.03 |
| Error | 1.702 | 75 | 0.023 | |||
| Total | 205.583 | 82 | ||||
| Corrected Total | 1.973 | 81 |
Table 3: Factorial ANOVA analysis of the main effects for log-transformed total SCFAs mean differences (μmoles/g stool).
This study looked into the relationship between fecal SCFAs and intestinal inflammation. Previous researches have revealed that the SCFAs such as acetate, butyrate, and propionate, each with a distinct degree of function, can reduce intestinal inflammation [8,12,34]. In several other researches, an increase in the amount of SCFAs in the gut has been identified as a functional biomarker of the helpful bacteria that lives there and lowers gut inflammation [35]. Butyric acid was discovered to have a suppressive effect on lymphocyte activation in a Chinese investigation on intestinal metabolites and childhood undernutrition. The results of the present study revealed that there was a weak and negative association between the concentration of total SCFAs and gut inflammation, which was a similar finding with the previous reports. According to the findings of Cong and his colleagues, Short-chain Fatty Acids (SCFAs) derived from the microbiota increase the production of IL-22, which is critical for gut immunity by CD4+ T cells and ILCs through inhibiting Histone Deacetylase (HDAC) and Gprotein Receptor 41 (GPR41) [36]. The weak association in the present study might be due to the small sample. It has been known that a sample that is either too small or too large could make it difficult to extrapolate the results and highlight statistical differences that are not clinically relevant [37].
With regard to the gut acidity, children with inflamed gut had less acidic status than their counterpart. There was a negative correlation between a colon’s pH and the total SCFAs. It was pointed out in an intervention study that probiotics increase Short-chain Fatty Acids (SCFA) and therefore lower the pH of the colon's environment, keeping pH-sensitive or pathogenic bacteria like Enterobacteriaceae and Clostridia out of a healthy colon and enhancing nutrient absorption [38]. Another study also demonstrated that the generation of acidic stools is positively influenced by Bifidobacteria and butyrate-producing commensal Clostridia, and a higher fecal pH suggests a decrease in these species in the gut [39]. Fecal pH and SCFAs were employed as test markers in a study that compared the gut microbiota of stunted and not stunted children in South India. As a result, the diversity of the gut microbial population is strongly correlated with both fecal pH and SCFA levels [39].
In the analysis of determinants of SCFAs, children who had used antibiotics and those who had a diet with little variety had significantly lower amounts of fecal SCFAs. It has been demonstrated that exposure to antibiotics has a negative impact on the healthy gut microbiota [40,41] which causes a decrease in the vital metabolites produced by the gut microbiota, notably SCFAs [42]. In the previous investigations, the timing of exposure, especially during the first three years of life, and the antibiotic spectrum level are both related to how much of an impact antibiotic have on the gut flora [43,44]. However, the specific age of exposure and the type of antibiotics that the children were exposed to were not covered by our study. On the other hand, dietary influence on the gut microbiota was suggested as the source of the microbiota as well as the substrate for the growth and metabolic processes [44,45]. In response to the properties of the diet that a baby eats, the gut microbiota goes through numerous evolutionary stages during infancy. The fact that diet can change the variety of gut microbes as well as the composition of their metabolic products has a significant impact on how nutrition affects human health beginning at a young age [46,47]. Numerous studies have shown that dietary fiber [48,49]. which is primarily found in fruits and vegetables, is the source of SCFAs made in the colon. However, the present investigation found no connection between eating fruits and vegetables and the content of SCFAs. This may have something to do with how digestible or indigestible the fiber is as SCFAs are mainly produced from indigestible ones [50,51].
Gut inflammation was substantially correlated with fecal SCFAs. Low dietary diversification and exposure to antibiotics both had an impact on the quantity of fecal SCFAs. Fecal SCFAs and fruits and vegetables as dietary fiber sources did not significantly correlate. Based on these findings, we recommend that government policy concentrate on raising community awareness about proper antibiotic usage and enhancing the nutritional diversity of children under the age of five using the food options. A greater sample size and consideration of the kind of dietary fiber that children consume call for more investigation.
This study was conducted according to the guidelines laid out in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of Hawassa University (Ref. No.: IRB/086/13). Verbal informed consent was obtained from all subjects before data collection. Verbal consent was witnessed and formally recorded.
Citation: Lefebo BK, Kassa DH, Tarekegn BG (2023) Fecal Short-Chain Fatty Acids are Associated with Gut Inflammation among Children of under Five at Hawassa, Sidama Regional State, Ethiopia. J Food Microbial Saf Hyg. 8:189.
Received: 30-Mar-2023, Manuscript No. JFMSH-23-22720; Editor assigned: 03-Apr-2023, Pre QC No. JFMSH-23-22720; Reviewed: 17-Apr-2023, QC No. JFMSH-23-22720; Revised: 24-Apr-2023, Manuscript No. JFMSH-23-22720; Published: 01-May-2023 , DOI: 10.35248/2476-2059.23.8.189
Copyright: ©2023 Lefebo BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.