Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2024)Volume 15, Issue 2
Choroideremia (CHM), a rare chorioretinal dystrophy with an incidence of 1 in 50,000 to 1 in 100,000, is marked by progressive atrophy of the retina and choroid. Choroideremia is caused by mutations in the CHM gene, which encodes Rab Escort Protein-1 (REP-1), involved in vesicular trafficking. Often misdiagnosed as Retinitis Pigmentosa (RP), distinguishing them is crucial. We present a case of choroideremia initially misdiagnosed as RP, emphasizing the importance of genetic testing and fundus auto-fluorescence imaging. Accurate diagnosis is essential for proper genetic counseling and timely enrollment in emerging gene therapies. This case highlights the role of multi-modal imaging in guiding the diagnostic process for choroideremia.
Choroideremia; Retinitis pigmentosa; Gene therapy
Choroideremia (CHM) is an uncommon chorioretinal dystrophy, occurring at an incidence rate between 1 in 50,000 and 1 in 100,000 [1,2]. It follows an X-linked inheritance pattern attributed to mutations in the CHM gene on the Xq21.2 chromosome [3]. This condition is marked by affecting the photoreceptors, Retinal Pigment Epithelium (RPE) and choroid. Although the biochemical and genetic abnormalities in CHM are well understood, the resulting pathogenesis of the disease remains unclear. It is yet to be determined whether the degeneration of the retina, RPE and choroid occur independently or sequentially in a layer-by-layer fashion.
RP is the most common type of retinal dystrophy worldwide, which affects around 1 in 4,000 individuals [4]. RP has a highly diverse genetic basis and X-linked Retinitis Pigmentosa (XLRP), in particular, is mainly correlated with mutations in the Retinitis Pigmentosa Guanosine Triphosphate (GTPase) Regulator (RPGR) gene [5]. Choroideremia and RP share common clinical features, such as the gradual decline in visual acuity, night blindness (nyctalopia) and progressive visual field loss [6,7]. Due to their shared X-linked inheritance pattern, clinical diagnosis may be confusing [8-10]. Consider the clinical distinctions among female carriers of choroideremia and XLRP. While most female carriers of choroideremia generally maintain good visual function with uncommon significant impairment, experiencing either no symptoms or mild to moderate night blindness, female carriers of XLRP exhibit a broad spectrum of clinical features, spanning from mild or moderate abnormalities to severe disease [11-14].
We report a case of choroideremia misdiagnosed as RP, highlighting the importance of genetic testing and multi-modal imaging for diagnosing female carriers of choroideremia.
A 26-year-old Hispanic female was referred to our clinic due to peripheral retinal changes seen during a routine evaluation elsewhere and an initial diagnosis of RP. The patient reported no visual complaints and past medical and family history was unremarkable. The Best Corrected Visual Acuity (BCVA) on examination was 20/25 and 20/20 on the Oculus Dexter (OD) and Oculus Sinister (OS), respectively. Slit-lamp bio microscopy revealed a normal anterior segment examination in both eyes. Fundus examination of both eyes showed chorioretinal atrophy mid-peripherally and centrally, except for a portion of preserved tissue in the macula as shown in Figure 1. Fundus Auto- Fluorescence (FAF) imaging showed a speckled pattern of low and high-density shown in Figure 2. Optical Coherence Tomography (OCT) of both eyes was normal (Figures 1 and 2).
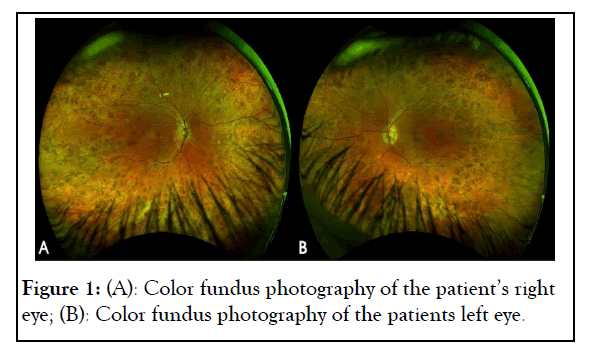
Figure 1: (A): Color fundus photography of the patient’s right eye; (B): Color fundus photography of the patients left eye.
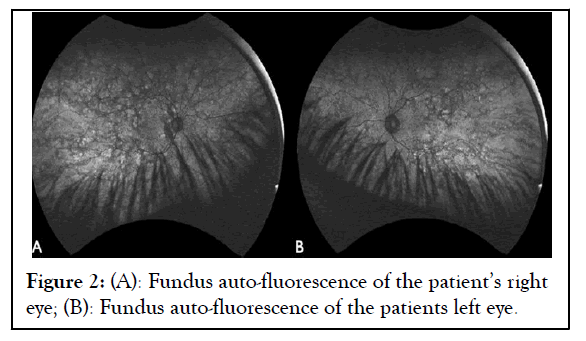
Figure 2: (A): Fundus auto-fluorescence of the patient’s right eye; (B): Fundus auto-fluorescence of the patients left eye.
Genetic studies showed a heterozygous pathogenic (deletion in exons 1-2) mutation in the CHM gene, leading to a CHM diagnosis as shown in Table 1. Additionally, the patient had a heterozygous pathogenic (c.1239+5G>C (intronic)) mutation in the Oculocutaneous Albinism (OCA) 2 gene. Invitae Corporation (IC) utilized Next-Generation Sequencing (NGS) with its genotyping microarray for mutational screening. A total of 330 genes were tested using genomic Deoxyribo Nucleic Acid (DNA) extracted from a blood sample following standard protocols. The Illumina technology was applied to sequence the specified regions by enriching genomic DNA from the provided sample through hybridization-based protocol (Figure 3 and Table 1).
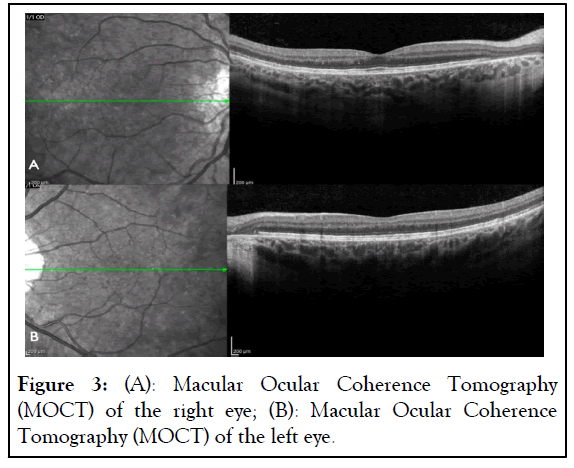
Figure 3: (A): Macular Ocular Coherence Tomography (MOCT) of the right eye; (B): Macular Ocular Coherence Tomography (MOCT) of the left eye.
| Gene | Variant | Zygosity | Variant |
|---|---|---|---|
| CHM | Deletion (exons 1-2) | Heterozygous | Pathogenic |
| OCA | c.1239+5G>C | Heterozygous | Pathogenic |
Note: CHM: Choroideremia; OCA: Oculocutaneous Albinism.
Table 1: Genetic studies showing heterozygous pathogenic (deletion in exons 1-2) mutation in the CHM gene.
Female carriers typically show minimal symptoms, although minor retinal signs such as pigmentary changes may be evident during fundus examination. Their condition can vary in severity, but they are generally asymptomatic. In contrast, affected male patients experience nyctalopia, progressive peripheral visual field loss and reduced central visual acuity. Central visual acuity typically declines from the fourth decade of life [15].
In the initial assessment of patients suspected of having an inherited retinal dystrophy, easily accessible tools can be employed, with fundus auto-fluorescence linked to lipofuscin deposits in RPE cells. Fundus auto-fluorescence is a proxy for lipofuscin distribution in the RPE and has evolved into a critical biomarker for tracking disease progression in choroideremia. The varied FAF patterns in the central fundus depend on the inherited disease's stage and type. Consequently, FAF imaging is a non-invasive diagnostic tool helpful in identifying female carriers of X-linked inherited retinal dystrophy, especially when small changes in RPE lipofuscin content are not discernible through ophthalmoscopy [16,17].
Fundus Auto-Fluorescence (FAF) has the potential to uncover abnormalities not identifiable through funduscopic examination, Fluorescein Angiography (FA) or Optical Coherence Tomography (OCT). In FAF imaging, female carriers of choroideremia exhibit a distinct speckled pattern of low and high density, a feature unique to this condition and absent in other inherited retinal diseases [18]. Similarly, female carriers of X-linked Retinitis Pigmentosa (XLRP) display alterations in FAF patterns, including a radial pattern, not found in other inheritance patterns of RP [19]. The contrasting FAF patterns in these diseases establish FAF imaging as a potent clinical tool for precisely diagnosing X-linked chorioretinal disorders. While genetic testing is increasingly accessible for definitive diagnosis, FAF imaging holds particular value in guiding this diagnostic process.
Genetic testing holds significance for choroideremia patients frequently misdiagnosed as having RP, as the CHM gene is uniquely linked to choroideremia, encoding the Rab Escort Protein-1 (REP-1) involved in intracellular vesicular trafficking. REP-1 deficiency leads to progressive chorioretinal degeneration, resulting from accumulating unprenylated proteins in the cytosol due to the loss of REP-1 functions, ultimately causing cellular dysfunction and premature cell death [20]. Presently, over 280 mutations within the CHM gene have been documented with choroideremia, primarily consisting of point mutations that directly introduce premature stop codons. This study identified a novel mutation in the CHM gene with deletions in exons 1 and 2.
Choroideremia, although rare, presents distinct clinical and genetic characteristics that differentiate it from more prevalent conditions like retinitis pigmentosa. This case underscores the need for a comprehensive approach to diagnosing inherited retinal dystrophies, incorporating genetic testing and advanced imaging techniques such as fundus auto-fluorescence. Utilizing FAF imaging is crucial for distinguishing female carriers of XLRP from choroideremia, as it reveals a distinctive pattern facilitating clinical diagnosis. The characteristic speckled pattern observed in FAF imaging of female carriers of choroideremia serves as a crucial diagnostic marker, aiding in accurate differential diagnosis. This imaging technique relies on observing intrinsic fluorophores and can be readily and swiftly applied in routine patient care. Molecular testing is essential for comprehensive genetic counseling, enabling the identification of female carriers. Furthermore, the timely and accurate diagnosis of choroideremia is pivotal in enrolling patients in ongoing gene therapy developments.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hernandez-Emanuelli ME, Davila PJ, Izquierdo NJ (2024) Female Choroideremia Carrier: Importance of Genetic Testing and Imaging in Accurate Diagnosis. J Clin Exp Ophthalmol. 15:975.
Received: 26-Feb-2024, Manuscript No. JCEO-24-30126; Editor assigned: 28-Feb-2024, Pre QC No. JCEO-24-30126 (PQ); Reviewed: 13-Mar-2024, QC No. JCEO-24-30126; Revised: 20-Mar-2024, Manuscript No. JCEO-24-30126 (R); Published: 28-Mar-2024 , DOI: 10.35248/2155-9570.24.15.975
Copyright: © 2024 Hernandez-Emanuelli ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.