Medical & Surgical Urology
Open Access
ISSN: 2168-9857
ISSN: 2168-9857
Research Article - (2015) Volume 4, Issue 3
Azoospermia is defined as the absence of spermatozoa in the semen. If no spermatozoa are observed in the wet preparation, the World Health Organization (WHO) recommends an examination of the centrifuged sample (3000 X g or greater for 15 minutes). Approximately 1% of all men in the general population suffer from azoospermia, and azoospermic men constitute approximately 10 to 15% of all infertile men. This work aims to investigate whether color Doppler ultrasound can detect active spermatogenic foci within azoospermic testis or not. Also to evaluate the technique of FNAC as an alternative method for qualitative and quantitative analysis compared with open testicular histopathology. This work was carried out on 150 patients, complaining of infertility for at least one year. In conclusion, the results of this study demonstrate that color Doppler sonography represent promising methods for the assessment of patients affected by azoospermia allowing us to discriminate obstructive azoospermia (normal vessel distribution) from non-obstructive azoospermia (reduced or absent testicular vessels). FNAC of azoospermic testis is a cost-effective and safe method of evaluating male infertility.
<Keywords: Spermatogenic; Sonography; Infertility; Azoospermia
Azoospermia is defined as the absence of spermatozoa in the semen. If no spermatozoa are observed in the wet preparation, the World Health Organization (WHO) recommends an examination of the centrifuged sample (3000 X g or greater for 15 minutes). If no sperm are observed in the centrifuged sample, the semen analysis should be repeated. The presence of a small number of spermatozoa in either of the centrifuged samples is defined as cryptozoospermia, and the complete absence of spermatozoa is defined as azoospermia [1].
Approximately 1% of all men in the general population suffer from azoospermia, and azoospermic men constitute approximately 10 to 15% of all infertile men. Azoospermia frequently represent the endpoint of pathological conditions that causes important quantitative and qualitative alteration of both spermatogenesis and testicular structure, including intratesticular blood supply [2].
Azoospermia is classified as obstructive and non-obstructive. Obstructive azoospermia is characterized by a testicular biopsy demonstrating sufficient spermatogenesis and a physical occlusion of the reproductive tract distal to the testis that prevents sperm from entering the semen. Non obstructive azoospermia (NOA) is caused by severely reduced sperm production, resulting in the absence of sperm in the semen [3].
The blood supply to testis is either nutritional or functional blood vessels. The nutritional blood vessels are equal in both obstructive and non-obstructive azoospermia (the supratesticular vessels that supply feeding nutrition for testis and epididymis) [4].
The functional blood vessels (intratesticular) suggest the presence of residual spermatogenic areas in non-obstructive azoospermia especially at peripheral zones, so, it is different in obstructive azoospermia as there is a normal area of spermatogenesis [5].
The color Doppler ultrasound imaging evaluates the intratesticular blood flow and it has been proposed as a promising method for the assessment of patients with intrascrotal pathological conditions [6].
Also to evaluate if testicular blood flow is impaired in the presence of a primary testicular pathology or not and whether its distribution makes it possible to identify active spermatogenic foci or not [2].
Several methods of aspiration biopsies of the testis were proposed in the past as a less invasive way to obtain materials for histologic or cytologic evaluation but did not receive enough clinical acceptances because it was considered to be too traumatizing. Afterward fine needle aspiration (FNA) of the testis was proposed as a non-invasive technique but did not enter in the routine diagnostic procedures as the cytological analysis of germinal cells as seen in the cytological smears, show different features from those observed in histologic section [6].
In this study the Doppler guided fine needle aspiration cytology is performed as a new method of detecting active spermatogenic foci that may help in both diagnosis and treatment (ICSI). Fine needle aspiration cytology of the testis in azoospermic men is a reliable and informative method to assess spermatogenesis [3].
This work aims to investigate whether color Doppler ultrasound can detect active spermatogenic foci within azoospermic testis or not.
Also to evaluate the technique of FNAC as an alternative method for qualitative and quantitative analysis compared with open testicular histopathology.
This work was carried out on 150 patients, complaining of infertility for at least one year. The azoospermic patients were selected from the Andrology unit of Dermatology, Venereology and Andrology Department, Zagazig University Hospital, along more than two years. Their ages ranged from 18 to 54 years.
Each patient was subjected to thorough history taking and examination according to the following scheme:
Carful history is taking of the duration of the couple’s infertility and previous evaluation and treatment of either partner.
Testicular volume was evaluated by using the approximation of volume = K × abc, where K is constant, equaling 0.523 and abc are the three major testicular axes [5].
All azoospermic patients underwent two semen analyses and should be performed according to the World Health Organization recommended procedure in the following (Table 1) [7].
| *Normal seminal fluid analysis (World Health Organization, 2010) | |
| • Volume | 1.5 ml |
| • Sperm concentration | 15 million/ml |
| • Sperm motility | >32% progressive |
| • Morphology (strict criteria) | >4% normal forms |
| • White blood cells | <1 million/ml |
| • Immunobead or mixed antiglobulin reaction test* |
<10% coated |
Table 1: Tests for the presence of antibodies coating the sperm.
Plasma concentration of FSH (normal level 1-9 mIU/ml) and LH (normal range 1.5- 9.2 mIU/ml) were measured by radioimmunoassay.
The most important hormonal measurement in the azoospermic male is serum FSH level for both the diagnostic and prognostic point of view [8,9].
Doppler sonography performed by a 7.5 MHz phased-array transducer. Testicular volume was evaluated by using the approximation for a prolate ellipsoid (volume = length X width X depth X O.523). Any fibrosis within testicular tissue was detected [5].
The Doppler also identified intratesticular arteries (functioning arteries in peripheral testicular zone). In all patients, scans were obtained in multiple orientations to ensure the imaging of blood vessels. The analysis of intratesticular blood vessels per organ was quantified using a semi quantitative score: category 0, no visible vessels; category 1, between one and three intratesticular vessels visible; category 2, more than three vessels could be identified. It was also determined whether visible intratesticular vessels were located in the cranial, middle, or caudal third of the testis to ensure the site of aspiration [5].
As these sites of functional arteries in peripheral testicular zone are markers of active spermatogenic foci within azoospermic testes FNAC obtained from it [5].
Patients have been done open testicular biopsy (with histopathological examinations), before attending our outpatient clinic and hence, these patients are considered as control cases to evaluate the efficacy of fine needle aspiration cytology versus histopathology.
FNA is an outpatient procedure performed in a warm room to relax the scrotal skin. Before this procedure, every patient writes consent for doing this maneuver. Patients were given Atropine ampoule intravenously to block the vasovagal reflex, 15 minutes, before spermatic cord block.
After an aseptic painting of the scrotal skin, the spermatic cord is infiltrated with 5 mL of 0.5% Xylocaine using 25-gauge needle attached to 5ml syringe, at each side. After 20 minutes, the testis is palpated to ensure absence of pain and positioned with the epididymis and vas deferens directed posteriorly away from needle injury. The scrotal skin is stretched over the testis by elevating the testis and circumferentially wrapping the scrotal skin behind the testis with gauze. The ‘testicular wrap” serves as a convenient handle to manipulate the testis and also fixes the scrotal skin over the testis [10].
According to the Doppler finding the site of aspiration is detected according to high vascularity in each testis [11].
FNA is performed with a sharp-beveled, 23 gauge fine needle attached to a 20 ml syringe. The needle is percutaneousely placed into the testis at sites detected by color Doppler. Suction is then applied and the syringe holder held steady as the needle is moved in-and-out, without changing direction and keeping the needle tip within the substance of the testis.
The suction is released before the needle is withdrawn from the testis. The needle disconnected from the syringe, and then it was evacuated on glass microscopic slide, the needle reattached and the tissue expelled from the needle onto the same slide. The aspirated materials including seminiferous tubules are gently smeared on the slide and immediately fixed by 95% ethyl alcohol [5].
The needle and syringe are changed for each aspiration side. Pressure is applied to the aspiration sites for several minutes for homeostasis. May-Grunwald-Giemsa stain is performed on the slides which are then examined under light microscope [12].
Using the differential cell count method each smear was screened for number and shape of germ and sertoli cells (normally at least 200 cell/ smear) defect in number of germ and sertoli cells classified to; cellular >100, hypocellular 50-100 and acellular <50), with 400X (high power) light microscope [13,14].
In normal spermatogenesis spermatozoa must = 45% of germ cells or 10-20 spermatozoa / 400X [10].
The shape of sertoli cells may be normal or distorted (in fibrosis and hyalinization) [14].
The shape of seminiferous tubules may be long (normal), short fibrosed (peritubular fibrosis) or hyalinized fragments [14].
Regarding the cellularity the aspirates were divided into a) acellular: no germ cells in the aspirate or very few germ cells from which no relevant data can be obtained, b) hypocellular: from 50-100 germ cells in the aspirate and c) cellular: more than 100 germ cells founded in the aspirate, (Figures 1-3).
How to comment on the state of the basement membrane and shape of sertoli cells? (Figure 4).
-Long, serpiginous fragments of tubules (normal basement membrane) are seen in aspirate of normal testis.
-Normal round or oval sertoli cells denoting no peritubular fibrosis/ hyalinization.
The deferential cell count of FNAC presented according to the following report (Table 2).
| Testicular cells | Rt. Testes | Lt. Testes |
|---|---|---|
| Total count | = /smear | =/smear |
| -Germ cells: -Spermatogonia -1ry spermatocyte -2ry spermatocyte -spermatid - round - late -spermatozoa |
= /smear | = /smear |
| -Sertoli cells | ||
| -Seminiferous tubules |
Table 2: Fine needle aspiration cytological report of testis.
Dermatology, Venereology and Andrology Depatment
Zagazig University Hospital
Andrology Unit
Fine needle aspiration cytological report of testis
Name :………………………………
Age :………………………………
Date :……………………………
Referral :…………………………
Stain: -Giemsa stain -Other stains
Site :……………………………
Conclusion :……………………
Sign :
After aspiration, patients were given antibiotics to guard against infection and analgesic as pain killer.
Data were coded, entered and analysed using epi-info (2000). Data were presented as mean ± standard deviation for quantitative variables, number and percentage for qualitative variables.
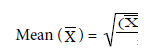
Where Σ = sum
X = observation value

Student T-test: used for comparison of two means:
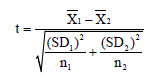
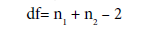
where = Mean
SD= Standard deviation
n= number of observation
df= degree of freedom
Chi-squared (X2) test: used to find the association between row variable and column variable
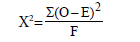
df= (r-1) (c-1)
Where O= observed value

R = row
C = column
(Table 3)
| Diagnostic | ||
| + ve | - ve | |
| Screening | ||
| + ve | A | B |
| - ve | C | D |
Table 3: Validity.
a= true+ve b=false+ve
c=false-ve d=true-ve
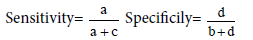
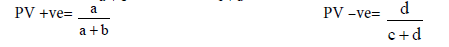
In this study, 150 azoospermic patients were included and FSH level was estimated for all patients. Doppler guided FNA were done for all patients.
The age of patients included in this study ranged between 18 and 54 years (mean 36).
One hundred and thirty three patients were complaining of primary infertility while only 17 patients were complaining of secondary infertility.
The duration of infertility ranged from a minimum of 1 year to a maximum of 30 years (mean 15.5).
In this study there were 32 patients having small testicular size (< 8 ml), 20 patients showed moderate testicular size (8- 15), while 96 patients had normal testicular size (18-20 ml). Two patients were having Rt. Undescended testis.
Serum FSH level was estimated for all patients. The minimum level was 1.5 and the maximum level was 112 (mean 56.3). They were classified according to level of FSH into: normal (1- 9) = 65 patients, increased level of FSH (9- 18) = 35 patients and excessively increased level of FSH (> 18) = 50 patients (Table 4).
| Group | Value | No. of cases | Percent |
|---|---|---|---|
| Normal Increased Excessively increased |
1.5 - 9 9 - 18 > 18 |
65 35 50 |
43.33% 23.33% 33.33% |
| Total | 150 | 100% |
Table 4: Classification of patients according to FSH level.
The correlation between FSH and testicular size are shown in (Table 5).
| FSH level | Small testes | moderate | normal |
|---|---|---|---|
| Normal Increased Excessively increased |
4 9 50 |
4 23 22 |
120 35 28 |
| Total (295 testis) | 63 | 49 | 183 |
Table 5: Showing correlation between FSH level and testicular size.
In this study 64 patients were given -ve Doppler image of functioning blood vessels. While 86 patients give +ve Doppler image of functioning blood vessels, (39 of them showed > 3 functioning arteries and 47of them were < 3 functioning arteries) (Table 6), (Figures 5-8).
| No. of arteries in image | Bil. Doppler findings |
|---|---|
| % | |
| > 3 functioning arteries | 25.7% |
| < 3 functioning arteries | 31.8% |
| No functioning arteries | 42.37% |
| Total | 100% |
Table 6: Showing the Doppler finding in both testes.
Fine needle aspiration
Fine needle aspiration cytology was done for all patients included in this study. The interpretation of smears was done according to differential cell count method.
The pattern of FNAC smear, the cellularity, the shape of sertoli cells and the state of seminiferous tubule in Azoospermic testes of this work were illustrated in (Table 7) and (Figures 1-3).
| Classification | percent |
|---|---|
| Acellular = 0-50 Hypocellular = 50-100 Cellular > 100 cells |
33.9% 48.1% 17.9% |
| Total | 100% |
Table 7: Cellularity of the FNA.
The correlation between Doppler finding (number of functioning arteries within the testis) and the reading of the cytological smear are shown in (Table 8).
| Percent of cases | Seminiferous tubules | Cellurality | Pattern |
|---|---|---|---|
| 12.88% | Long serpiginous | High | Normal spermatogenesis |
| 12.88% | Long serpiginous | Moderate and occasionally high | Hypospermatogenesis |
| 40.33% | Straight fragments | moderate | Spermatogenic Arrest at: - Late spermatid - Round spermatid - 1ry spermatocyte |
| 26.44 % | Short frag./ fibrous tissue | Acellular | Sertoli cell only |
| 3.38% | Absent/ fib. Tissue | Acellular with distorted sertoli cells | Hyalinization &peritub.fibrosis |
| 4.06 % | Short frag./ fibrous tissue | Acellul./some distorted sertoli cells | Mixed lesions |
| 100% | Total |
Table 8: Show the patterns of testicular FNA cytology.
In this work, patients had done open testicular biopsy before attending the outpatient clinic, 110 of them correlated histopathologically with finding of FNAC and the remaining 40 not correlated Histopathologically with FNAC findings (Table 9).
| P.value | No arteries | <3 arteries | >3 arteries | Pattern | |||
|---|---|---|---|---|---|---|---|
| % | testes | % | testes | % | testes | ||
| <0.001 | -- | -- | 19.1% | 18 | 26.3% | 20 | Normal spermatogenesis |
| <0.001 | 1.6% | 2 | 17% | 16 | 26.3% | 20 | Hypospermatogenesis |
| 0.55 N.S. | 39.2% | 49 | 44.7% | 42 | 36.8% | 28 | Spermatogenic arrest |
| <0.001 | 49.6% | 62 | 10.6% | 10 | 7.9% | 6 | Sertoli cell only |
| <0.001 | 8% | 10 | -- | -- | -- | -- | Hyalinization and peritubular fibrosis |
| 0.054 N.S. |
-- | -- | -- | -- | 2.6% | 2 | Mixed lesions: Hypo + others MA + others SCO + others |
| <0.001 | -- | -- | 8.5% | 8 | -- | ||
| 0.25 N.S. |
1.6% | 2 | -- | -- | -- | ||
| P< 0.05 | 100% | 125 | 100% | 94 | 100% | 76 | Total |
Table 9: Showing the correlation between Doppler and cytology findings.
General complication of vasovagal attack detected in 2 patients out of 150.
Local complication in the form of hematoma that appeared 0.5- 2 hours after aspiration was detected in 15 cases that resolve within 1 week, post aspiration pain (1-3 hours) was detected in 75 patients that resolve within 1 day. Difficult aspiration was detected in cases of hydrocele and small atrophied testis (Table 10).
| Biopsy histology | No. of patients |
|---|---|
| -Normal -Hypospermatogenesis -Spermat. Arrest -Sertoli cell-only -Hyalinization and peritubular fibrosis. |
30 40 50 20 10 |
| Total | 150 |
Table 10: Showing the differential cell counts of FNAC (Doppler guided) in correlation to open testicular biopsy.
Testicular ultrasound was done for patients 3months post aspiration (to detect residual fibrosis), minimal fibrosis was detected in 2 cases but others showed no evidence of fibrosis.
The most severe expression of male factor infertility is azoospermia, where no sperm are present in the ejaculate. In this study azoospermia was found in about 35% of men evaluating for infertility [6].
The explanation of high incidence of azoospermia between infertile men is unknown as we have only 14 patients from 150 with relevant history related to azoospermia (e.g. genital T.B., trauma to testis).
However the high incidence of Bilharzias in Egypt may be the cause of many cases of obstructive azoospermia. Also environmental pollution may play a role in high incidence of functional azoospermia [2].
Testicular biopsy was the main diagnostic tool in the evaluation of male infertility since 1940s. However the invasiveness of the biopsy has limited its use and acceptance to the clinician as well as to the patient, especially when it is done for diagnostic purpose only (it is accepted only if it is done for both diagnosis and ICSI or semen banking at the same setting) [14].
Fine needle aspiration of the testis, was proposed as a noninvasive technique for diagnosis of azoospermia but it did not enter in the routine diagnostic procedures probably for these reasons: 1) the difficulty of cytological analysis of germ cells because isolated cells in cytological smear show different features from those in histological section, 2) cytological specimens give no information about tubular diameter, thickness of the tubular basement membrane or status of the interstitial tissue [2].
With the new paradigm of treatment option of azoospermia, what we require from the diagnostic testis biopsy has also changed. Because both normal and failing testes may undergo biopsy routinely, any technique to assess spermatogenesis must be minimally invasive and must conserve as much testis tissue as possible. Also many of the nonobstructive azoospermic testis are small atrophied not liable for further tissue biopsy [6].
The diagnostic techniques of azoospermia must also give information about spermatogenesis and whether any sperm are present at all within the testis. Although the testis biopsy has some prognostic value in predicting successful sperm recovery for ICSI, it may now be insufficient for modern male infertility care [6].
For these reasons, the use of testicular FNA cytology to assess spermatogenesis has been renewed. FNA cytology of the testis has been considered quick, safe and minimally invasive [10].
The use of Doppler guidance in fine needle cytology aspiration in this work gives good results than the blind testicular biopsy. In this study by good cytological interpretation of germ cells and other testicular cells (from the available literature and reference books), the cytological analysis of testicular cells become easy and simple.
In conclusion, the results of this study demonstrate that color Doppler Sonography represent promising methods for the assessment of patients affected by azoospermia allowing us to discriminate obstructive azoospermia (normal vessel distribution) from nonobstructive azoospermia (reduced or absent testicular vessels). Furthermore, the findings of this study, suggest that the presence of blood vessels, especially in peripheral regions, may indicate the possible presence of residual spermatogenic foci.
FNAC of azoospermic testis is a cost-effective and safe method of evaluating male infertility. Because the differential cell counts in smear correlate well with different histological categories. Its use may help in correct classification of the condition and also in quantitation of spermatogenesis, that lead to the correct choice of therapy.
The use of FNAC may obviate the need for other costive, highly invasive methods. It is recommended to use Doppler before any aspiration to minimize and locate sites of aspiration. Also in TESE the color Doppler may provide a help in detecting which site is suitable for sperm extraction for ICSI. FNAC can be repeated after hormonal replacement therapy to follow up the maturation of germ cells, without fear of complications.