Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
+44 1478 350008
ISSN: 2329-8790
+44 1478 350008
Research - (2023)Volume 11, Issue 3
Background: Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare acquired genetic hematopoietic stem cell disorder due to a somatic mutation of the X-linked Phosphatidyl-Inositol Glycan (PIG-A) gene. It is closely related to aplastic anemia with a significant number of aplasia patients having a PNH clone.
Methodology: A total of 115 aplastic anemia patients were enrolled in the study. PNH testing was done by multiparameter flow cytometry analysis specifically using Fluorescein-Labeled Proaerolysin (FLAER) to increase the sensitivity of the procedure. The antibody panel for neutrophils and monocytes consisted of CD45, CD15, CD157 and CD64 along with FLAER. Whereas, CD235a was used for gating the true RBCs population on which the co expression of CD59 was observed. This population was differentiated into RBCs with complete and partial deficiency based on CD59 expression.
Results: A total of 70 (60.9%) out of 115 enrolled patients were detected to have PNH clone. There were 80 (69.6%) male and 35 (30.4%) female. Mean age of the patients was 33.63 Â ± 16.78 years. Out of these patients 65 (56.5%) patients had Non Severe Aplastic Anemia, while 36 (31.3%) and 14 (12.2%) were classified as Severe Aplastic Anemia and Very Severe Aplastic Anemia respectively. The mean bone marrow cellularity was 13.19% Â ± 7.89%. A very small PNH clone was found in 23 (20.0%) patients while 35 (30.4%) had a small PNH clone size and 12 (10.4%) patients had a large PNH clone size.
Conclusion: Aplastic anemia patients have a relatively high frequency of PNH Clone of varying size when assessed with sensitive technique like MPFC using FLAER.
Aplastic Anemia; Paroxysmal nocturnal haemoglobinuria; Fluorescein Labeled Proaerolysin (FLAER)
Paroxysmal Nocturnal Haemoglobinuria (PNH) is a rare acquired genetic hematopoietic stem cell disorder with an annual rate of 1-2 cases per million [1]. The defect is due to a somatic mutation of the X-linked Phosphatidyl-Inositol Glycan (PIG-A) gene that results in partial or complete deficiency of Glycosylphosphatidylinositol (GPI) anchored proteins from cell surfaces. This deficiency makes the cells prone to complement mediated lyses resulting in hemolysis, bone marrow failure and frequent episodes of thrombosis [2,3].
Aplastic anemia, PNH and other bone marrow failure syndromes are pathogenically related, with many cases of PNH developing in patients who have previously been diagnosed as Aplastic Anemia (AA) and Myelodysplastic Syndrome (MDS) [4,5]. The presence of a PNH clone in AA patients has been explained by the presence of self-reacting GPI-specific T cells in AA and PNH patients. Thus an immune-mediated destruction by these T-cells would spare the GPI anchored proteins deficient cells giving rise to a PNH clone [6]. Traditionally, Ham’s test and sucrose lysis test were used to diagnose PNH but these tests soon became redundant as they were labour intensive and had low sensitivity and specificity [7,8]. Over the past several years, evaluation of GPI-anchored proteins on Red Blood Cells (RBC) and granulocytes by Multiparameter Flow Cytometry (MP-FCM) has become the gold standard. Flow cytometry based assays are more sensitive and specific than complement-mediated lysis or the Hams test, but initially they suffered from few drawbacks. The CD55/Decay Accelerating Factor (DAF) and CD59/ Membrane Inhibitor of Reactive Lysis (MIRL) based approaches were inadequate to detect small PNH clones (especially below 1%-2% clone size) in most PNH positive AA and MDS cases. Recently, more sensitive approaches have been developed for PNH detection based on the bacterial toxin Fluorescein Labelled Proaerolysin (FLAER). FLAER binds the GPI portion of the GPI anchored proteins increasing the specificity of detection. It is now increasingly been used to detect GPI-deficient leukocytes by flow cytometry as it has is not affected by recent transfusions and congenital deficiencies of GPI anchored antigens like CD55.
PNH clone in Chinese population with AA and MDS has been reported in up to 50% and 10-20% respectively [9]. In western population, PNH clone is detected in 50% to 60% patients with aplastic anemia [10]. In Indian population, the percentage is around 41% [11]. However, it was found to be 12.02% in a study conducted by MUS Sachdeva et al. on a large number of Indian patients with aplastic anemia [12].In PNH patients, these clones are linked to hemolysis and/or thrombosis, whereas the presence of rare GPI-deficient cells (0.1%) in aplastic anaemia and hypoplastic myelodysplastic syndrome is linked to a better response to immunosuppressive therapy, a lower risk of transformation to MDS, and/or acute myeloid leukaemia [13]. It is also believed that presence of PNH clone in an aplasia patient can be used as for the diagnostic exclusion of Inherited Bone Marrow Failure Syndromes (IBMFS) [14,15]. Cases with unexplained isolated anaemia, unclear iron deficiency anaemia, unexplained isolated neutropenia or thrombocytopenia, any subtype of MDS with evidence of hemolysis, venous or arterial thrombosis with atypical location and signs of hemolysis, cytopenias in a young patient, as well as acquired Coombs negative hemolysis, intravascular hemolysis, or recurrent abdominal pain or dysphagia were recommended indications for GPI‐AP deficiency testing [16].
In a large epidemiological study on 1324 AA patients from Pakistan the incidence of PNH clone in AA was reported as 6.7% [17,18]. However, PNH screening in these patients was based on testing the RBCs for presence of CD55 and CD59, which could explain the very low incidence of PNH clone in AA patients in Pakistan as compared to other countries. The purpose of this study was to perform FLAER based MP-FCM assay to improve detection of minor populations of PNH clones in AA patients.
This study was conducted after taking approval from the Ethics and Research Committee of the Armed Forces Bone Marrow Transplant Centre, Rawalpindi, Pakistan in accordance with the current Helsinki Declaration. It was carried over a six-month period. The study design was descriptive, cross-sectional. Patients of all age groups with a confirmed diagnosis of AA on bone marrow examination were included in the study. An informed written consent was taken from the participants and/or their parents in pediatric patients. The underlying severity of AA was also determined based on modified Camitta Criteria [19]. Patients receiving immunosuppressive therapy for AA or those with history of RCC transfusion within the last 10 days were excluded from the study.
Paroxysmal nocturnal haemoglobinuria testing
All patients underwent detailed medical history and full physical examination followed by blood sampling in EDTA tube for FLAER based MP-FCM assay. A minimum of 2 ml venous blood was extracted. Clotted blood or old samples (more than 48 hrs) were not processed. All the information such as age, sex, duration of disease, CBC, bone marrow examination and MP-FCM assay results was entered in a specifically designed proforma. The FLAER based MP-FCM was carried out in two tubes, one for the leukocytes and the other for RBCs. In the leukocyte tube FLAER was combined with CD45, CD15, CD157, and CD64 allowing the simultaneous analysis of FLAER and the GPI-linked CD157 structure on neutrophils and monocytes (Figure 1).
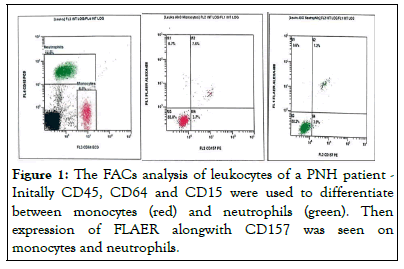
Figure 1: The FACs analysis of leukocytes of a PNH patient -Initally CD45, CD64 and CD15 were used to differentiate between monocytes (red) and neutrophils (green). Then expression of FLAER alongwith CD157 was seen on monocytes and neutrophils.
In the RBC tube, CD235a was used for gating the true RBCs population on which the co-expression of CD59 was observed. A threshold of 0.01% for clone size was set to be taken as positive. The population was further differentiated into RBCs subsets with complete and partial deficiency of CD59 expression (Figure 2).
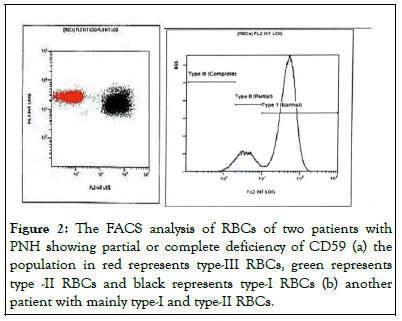
Figure 2: The FACS analysis of RBCs of two patients with PNH showing partial or complete deficiency of CD59 (a) the population in red represents type-III RBCs, green represents type -II RBCs and black represents type-I RBCs (b) another patient with mainly type-I and type-II RBCs.
Overall the PNH clone sizes were classified as very small when only 1% target population was GPI deficient; small, between 1% to 10 % and as large when more than 10% [14].
A total of 115 patients with diagnosis of AA were enrolled in the defined study. The study population comprised of 80 (69.6%) male and 35 (30.4%) female patients. The mean age of the patients was 33.63 ± 16.78 (range 3-77) years. Among the enrolled AA patients 65 (56.5%) patients were categorized as Non Severe Aplastic Anemia, while 36 (31.3%) and 14 (12.2%) were diagnosed with Severe Aplastic Anemia and Very Severe Aplastic Anemia respectively. The mean overall bone marrow cellularity of the patients was 13.19 ± 7.89 (range, 5-55) %. The demographics of the patients are shown below in Table 1.
| Categories | Frequency | Percent |
|---|---|---|
| Age wise distribution | ||
| 0-20 Years | 26 | 22.6 |
| 21-40 Years | 60 | 52.2 |
| 41-60 Years | 17 | 14.8 |
| >60 Years | 12 | 10.4 |
| Mean=33.63 ± 16.78 years | ||
| Sex wise distribution | ||
| Male | 80 | 69.6 |
| Female | 35 | 30.4 |
| Underlying severity of aplastic anemia | ||
| Non Severe | 65 | 56.5 |
| Severe | 36 | 31.3 |
| Very Severe | 14 | 12.2 |
| Overall bone marrow cellularity | ||
| 0%-20% | 45 | 39.1 |
| 21%-50% | 56 | 48.7 |
| >50% | 8 | 7 |
| Bone marrow iron stores | ||
| Present | 98 | 85.2 |
| Absent | 17 | 14.8 |
Table 1: Demographics of the enrolled aplastic anemia patients.
A total 70 (60.9%) patients out of 115 were detected to have PNH clone. As per the set criteria 23 (20.0%) patients were found to have a very small PNH clone size while 35 (30.4%) had a small PNH clone size and 12 (10.4%) patients were detected to have a large size PNH clone as shown in Figure 3.
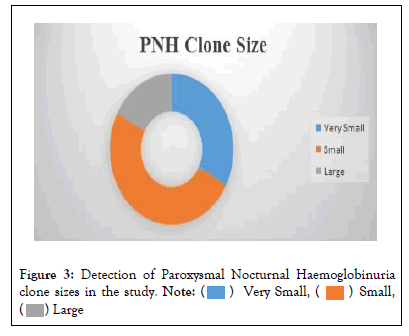
Figure 3: Detection of Paroxysmal Nocturnal Haemoglobinuria clone sizes in the study. 
Out of these 70 patients 60 (85.7%) had PNH clone in both the WBCs (neutrophils and monocytes) and the RBCs. The RBCs in the PNH clones were further stratified as type I, II or III and most of the patients showed type-I and type-II RBCs as shown in Table 2.
| Categories | Frequency | Percent | p-value | ||
|---|---|---|---|---|---|
| Age wise distribution | |||||
| 0-20 Years | 17 | 24.3 | 0.57 | - | |
| 21-40 Years | 36 | 51.4 | - | ||
| 41-60 Years | 11 | 15.7 | - | ||
| >60 Years | 6 | 8.6 | - | ||
| Gender wise distribution | |||||
| Male | 48 | 69.6 | 0.47 | - | |
| Female | 22 | 30.4 | - | ||
| PNH Clone in RBC and WBC | |||||
| Both RBC and WBC | 60 | 85.7 | - | ||
| RBC | 1 | 1.4 | |||
| WBC | 9 | 12.8 | |||
| RBC Type | |||||
| Types | Mean PNH clone size+SD | Range | |||
| Minimum | Maximum | ||||
| Type-I and Type-II | 48 | 68.6 | |||
| Type-II | 11 | 15.7 | 1.94+5.23 | 0.01 | 37.3 |
| Type-III | 2 | 2.9 | 6.5+12.99 | 0.001 | 48.1 |
| WBC Type | |||||
| Types | Mean PNH clone size+SD | Range | |||
| Minimum | Maximum | ||||
| Both Neutrophil and Monocyte | 54 | 47 | |||
| Neutrophil | 10 | 8.7 | 24.4+35.74 | 0.01 | 98.72 |
| Monocyte | 5 | 1.7 | 16.4+28.15 | 0.01 | 98.42 |
Table 2: Demographics of PNH clone positive patients (n=70).
A comparison of the PNH clone was done with the severity of AA, bone marrow cellularity and presence of iron store in positive cases using chi square test. The results are shown in Figures 4 and 5.
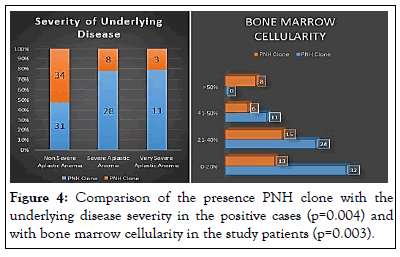
Figure 4: Comparison of the presence PNH clone with the underlying disease severity in the positive cases (p=0.004) and with bone marrow cellularity in the study patients (p=0.003).
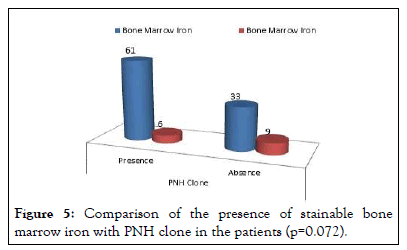
Figure 5: Comparison of the presence of stainable bone marrow iron with PNH clone in the patients (p=0.072).
The clinical and prognostic significance of a PNH clone in patients with aplastic anemia has been thoroughly analyzed and debated [20,21]. Its presence is indirectly used as a measure to differentiate between acquired immune-mediated aplastic anemia from inherited bone marrow failure syndrome and is associated with a better outcome to immunosuppressive therapy [22,23,24]. This is probably due to the detection of PNH clone in patients is reliably done by multiparameter flow cytometry. The International Clinical Cytometry Society (ICCS) guidelines recommend testing patients of AA and MDS for presence of a PNH clone [25]. The diagnosis is based on the 2 x 2 rule i.e. two different cell lines and deficiency of two different GPI anchored proteins. It more likely to be found in patients with AA as compared to those with MDS [26]. AA patients with a small PNH clone require serial monitoring as it may presage evolution to hemolytic PNH [27]. Incorporation of FLAER, a fluorochrome-conjugated proaerolysin that is activated by enzymes in the granulocytes and monocytes in MP-FCM increases the sensitivity and specificity of the detection. It binds directly to GPI anchor in the cell membrane and is able to detect a PNH clone as low as 0.01% in the leukocytes whereas, the traditional CD55 and CD59 based RBC assays had a lower limit of 1%-2% [28].
The mean age of patients in this study was 33.63 ± 16.78 years. These findings were consistent to the results of Park SH where he reported the same results pertaining to the age and duration of disease of patients. Using the high sensitivity method of detecting PNH clone we were able to identify 70 (60%) patients of AA with deficient GPI anchored protein. This is also in stark contrast to the previously reported 6.7% PNH clone presence in AA patients by Ahmed P et al. from our centre as at that time only CD55/ CD59 were tested on RBCs and most of the patients had recent transfusions[18]. It is slightly higher than the 50% frequency reported in Chinese as well as the Western population with AA [29]. No clear cut explanation is available for this relatively higher frequency as age and gender were not associated with the presence of PNH clone in the Pakistani patients with AA.
In this study, 65 (56.5%) patients had non-severe aplastic anemia, 36 (31.3%) patients has severe aplastic anemia, 14 (12.2%) had very severe aplastic anemia. The presence of the PNH clone was directly associated with the underlying severity of the disease (p=0.004). However, there was no direct comparison of the size of the PNH clone with the underlying disease severity (p=0.139). Similarly, it was found that patients with lower bone marrow cellularity had a higher chance of having a PNH clone (p=0.003). Interestingly, in spite of a high frequency of PNH clone presence, 99 (86.1%) patients had stainable bone marrow iron and absence of bone marrow iron had no statistically significant implication on finding the PNH clone in these patients.
Although chances of finding a PNH clone in monocytes are said to be more than neutrophils, it was not so in this study. The clone sizes in the neutrophils, monocytes, RBCs type-II and type-III ranged from 0.01% to 98.81%, 0.01% to 98.42%, 0.01% to 37.3% and 0% to 48.1%. This is slightly different from the clone sizes reported by S Sreedharanunni in a study from India with clone size in neutrophils and monocytes of 1.6% to 87.6% and 3.7% to 95.2% respectively [30]. However, his study was mainly focused on children less than 12 years of age. It is also important to remember that clone sizes are not constant and fluctuate over time in patients. A PNH clone size in access of 37% in RBCs and 28% in granulocytes has been set as threshold for onset of hemolysis in AA patients. As per treatment record, 87 (75.7%) had conservative management while 28 (24.3%) patients got immunosuppressive therapy (Table 2). These findings were consistent with the findings of Donohue RE where the results pertaining to BME opinion and treatment recorded had yielded almost the same results thus confirming our results. The previous studies from Pakistan reported PNH incidence based on CD55 and CD59 based testing; that might be the probable cause of lower incidence of PNH in aplastic anemia compared to the international data. Our findings were consistent with other reported literature. The major limitation of our study was the smaller sample size and lack of follow-up. The finding of >10% clone size in PNH cases and <10% clone size in majority of the aplastic anemia patients which needs to be validated in much larger studies and follow-up data should substantiate our findings.
This is the first study from Pakistan to use a standardized high-sensitivity FLAER-based multiparameter flow cytometry assay to identify PNH clone in patients with aplastic anemia. FLAER based flow cytometry assay can detect very small PNH clones less than 1% in monocytes and neutrophils even when no GPI anchored proteins are deficient in RBCs. Greater the severity of aplasia higher is the chances of finding a PNH clone in that patient. Combining both the RBCs and FLAER based leukocyte assays can identify PNH patients that were previously undetectable by flow cytometry for CD55/CD59.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Farooq Z, Shamshad GU, Ali Khan M, Yousaf M, Umar S (2023) FLAER Based Paroxysmal Nocturnal Hemoglobinuria Clone Detection in Patients of Aplastic Anemia: Single Centre Experience from a Developing Country. J Hematol Thrombo Dis. 11:536.
Received: 01-Mar-2023, Manuscript No. JHTD-23-21775; Editor assigned: 03-Mar-2023, Pre QC No. JHTD-23-21775 (PQ); Reviewed: 24-Mar-2023, QC No. JHTD-23-21775; Revised: 31-Mar-2023, Manuscript No. JHTD-23-21775 (R); Published: 07-Apr-2023 , DOI: 10.35248/2329-8790.23.11.536
Copyright: © 2023 Farooq Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.