Journal of Biomedical Engineering and Medical Devices
Open Access
ISSN: 2475-7586
ISSN: 2475-7586
Research Article - (2017) Volume 2, Issue 3
The present paper presents peak wall shear stresses and velocities in a bifurcated femoral artery. This artery is modeled along with blockage downstream of bifurcation, blockage at bifurcation, blockage downstream of stented femoral artery. The femoral artery is subjected to peripheral artery disease. A two-dimensional computational fluid dynamic analysis is conducted in all the models, assuming steady blood flow and seventy percent plaque. However, the blood viscosity and blood pressure vary in the femoral artery models as they are subjected to other medical conditions. Femoral artery bifurcates into profunda femoris and the bifurcation angles are assumed to vary at 30, 45 and 60 degrees. The blocked bifurcated artery is replaced with a wall stent and in many cases, a blockage develops downstream of the bifurcated stented artery as a consequence of stent implantation. The wall shear stresses and velocities results from the bifurcated and blocked femoral arterial models are compared to a normal artery characteristic. Further, a comparative study has been conducted along the blocked bifurcated artery with and without the stent.
Keywords: Dynamics; Atherosclerosis; Superficial femoral artery
Bilateral common iliac arteries are bifurcated from abdominal aorta. Iliac arteries are further branched into internal and external iliac arteries. Femoral arteries are part of external iliac arteries and extend to the lower extremities after passing under inguinal ligament. Further downstream, the common femoral artery branches off as profunda femoris [1], which are prone to atherosclerosis. Atherosclerosis commonly begins when plaque starts building up in the artery and atherosclerosis in the lower extremity it is called Peripheral Artery Disease (PAD). Peripheral artery disease is divided into asymptomatic and symptomatic disease [1]. PAD affects more than 12 million people in the United States and it increases with age [2-4]. Globally, 27 million people are affected by PAD and other atherosclerotic diseases [5,6]. Among the existing PAD patients, 20% of the patients suffer with intermittent claudication annually [2]. The number of discharges recorded per year based on chronic PAD are 413000 and 88000 patients get admitted in hospital who suffer with low extremity arteriography [3]. Studies have recognized an overlap between cerebral, coronary and peripheral artery disease. For instance, among patients with peripheral artery disease, there is sixty percent possibility of having coronary artery disease (CAD) and 35% percent possibility of having severe triple-vessel CAD with depressed ventricular function. Additional risk factors like smoking, diabetes, hypertension add to the existing problem [1]. Approximately 14 to 90% of the patients suffer with CAD and PAD. Cerebrovascular disease (CBVD) occurs in PAD patients and it is observed that 30% stenosis occurs in the carotid arteries [3]. However, some of the above mentioned problems like smoking, diabetes and hypertension are defined as inflammatory triggers. These triggers promote stress and enhance inflammatory paths either directly or indirectly. PAD is also observed commonly in 33% of monozygotic twin pair’s and 31% of dizygotic twin pairs due to heritability [6]. Lower Extremity PAD patients can be tested using noninvasive vascular laboratory tests and they are represented in Table 1.
| Clinical Problems | Non-invasive Vascular Test |
|---|---|
| Asymptomatic Lower Extremity PAD | ABI |
| Claudication | ABI, PVR, Segmental Pressures, Duplex Ultrasound, Exercise Test with ABI |
| Possible Pseudo claudication | Exercise test with ABI |
| Postoperative vein graft follow up | Duplex ultrasound |
| Femoral pseudo aneurysm, iliac or popliteal aneurysm | Duplex Ultrasound |
| Suspected Aortic Aneurysm, AAA follow up | Abdominal Ultrasound |
| Revascularization | Duplex Ultrasound, MRA, CTA |
Table 1: Non-Invasive Vascular Tests for Several Clinical Problems [28].
Femoral artery at bifurcated points are affected by wall shear stress in the presence of varying intima and media thickness. This issue is more effective in muscular arteries than elastic arteries hence an investigation was conducted non-invasively to observe the difference in wall shear stress with varying intima media thickness. Patients with age groups 21 to 74 were considered for the study in common (FC) and superficial femoral (FS) arteries. The maximum change in shear stress observed around the posterior wall did not have much effect on age groups. However, the intima media thickness increases with age [7,8]. Profunda femoris, medial and lateral femoral circumflex are the bifurcated arteries from the femoral artery. The origins of these bifurcations were studied using a case study and it suggests that lateral femoral and medial arteries are originated from femoral artery at low level of separation of profunda femoris [9]. A similar work was directed to examine the differences in the origin of circumflex and profunda femoris along with the measurement of the diameter of femoral artery [10]. In addition to this, the diameter of the femoral artery in children needs to be determined for cardiac catherization. From the study, the diameter could be determined based on the child’s age, body mass index (BMI) and height [11].
Femoropopliteal artery could deform during a knee flexion and hence a computational analysis was conducted to observe the mechanical behavior of a femoropopliteal intersection during knee bending. The study suggests the importance of material properties in tissue while determining the displacements of the femoropopliteal artery during knee flexion. Though, the study provided the approximation of stress levels in the femoropopliteal region, there are challenges associated with themodels like the mechanical properties of materials and the loads applied [12]. The shape of the femoral artery also has an effect on the flow simulations. Hence, a three-dimensional computational analysis was conducted to examine the wall shear stresses, pressure drop and velocity profile in an S shaped double curved femoral artery [13]. Superficial femoral artery (SFA) is affected by plaque that causes a decrease in the lumen area. A clinical analysis was conducted using magnetic resonance imaging that demonstrates a greater plaque area and smaller plaque lumen in PAD patients [14]. In rare situations, aneurysms occur in the common femoral artery. They sometimes cause thrombotic and embolic complications along with other connected aneurysms. The CFA aneurysms need screening and this can be achieved using duplex scan and post-operative follow up [15].
There are several ways to treat peripheral artery disease, out of which bare metal stents, balloon angioplasty and drug eluting stents play a major role. Besides their advantages, they also exhibit limitations which are analyzed using clinical, experimental and computational analysis. Protégé Ever Flex stent (Nitinol stent) is placed in the superficial femoral artery with peripheral artery disease. However, their long-term efficiency needs to be evaluated. Hence, a clinical study was conducted which shows that the Protégé Ever Flex stent has low fracture rate and less restenosis after 12 months from its implantation [16]. In contrast, self-expanding Nitinol stents cause fractures when they are placed in SFA and popliteal artery. The fractures cause disruption in the drug delivery system and may cause vessel injury. Hence, attempts are made to provide an optimum stent design and it is still in the ongoing process [17]. Similarly, a finite element analysis was conducted to determine the pre-existing crack in a self-expanding Nitinol stent. The study reports that, if the crack propagates 50 μm, it is a critical situation even if it does not fracture [18]. On the other hand, material properties of Nitinol stents are evaluated to observe if that could increase the mechanical performance. As part of the study, two different materials are tested and it shows that, high temperatures show better clinical behavior due to high radial strength and low outward force. When stents have high super elastic behavior, they exhibit better mechanical performance [19].
As bare metal stents suffer with limitations like restenosis in PAD patients, drug eluting stents (DES) are suggested. However, clinical studies show that DES has no additional benefits than bare metal stents in SFA. In contrast, the drug eluting stents are beneficial in popliteal artery because they show low restenosis rates after one year following from their implantation. In addition to this, the study also suggests that DES does not provide any additional benefits for balloon expandable stents in renal interventions [20]. As an alternative, drug eluting balloons can be used to prevent in stent restenosis. The clinical study indicates that it is a secure and effective surgical method to treat restenosis in SFA. However, this led to cardiac failure in some cases and hence more studies may be necessary on patients with comorbidities [21]. In succession to angioplasty, stent implantation and bypass graft, other examination techniques can be used to follow up the patients’ health condition. One such method is ultrasound technique in which femoral and popliteal arteries were studied. This method can afford a fuller sight of rare arterial variations, alterations in vessel morphology, information on atherosclerosis, lesion types and severity. However, there are limitations associated with this technique. The involuntary leg movements in patients, wall calcifications, color imaging and degree of arterial narrowing cannot be determined using this technique. If the examiner is not experienced there could be possibilities of error in velocity scale, the volume of the sample, Doppler angle and wall filters [22].
Considering the limitations associated with several techniques employed in treating PAD in superficial femoral artery, a review was conducted on the treatment methods for SFA lesions. The review explains the current challenges associated with endovascular revascularization in long term patency rates [23]. Similarly, a recent review suggests that endovascular treatment methods are secure and efficacious in treating lower extremity PAD. The study suggests latest technologies like local drug delivery, bio absorbable stents in order to avoid open surgeries [24].
Analysis of bifurcated femoral artery
Statistics show that patients with PAD are more likely to suffer from CAD, CBVD, cardiac and cerebrovascular disease (CCVD). In addition to these arterial diseases, many PAD patients are also affected by diabetes, hypertension and anemia [1]. These comorbidities needs further analysis. Risk factors associated with these conditions are predicted and clinical studies were conducted using the threshold of ankle brachial index. There are 50 to 400% chances of having CCVD in PAD patients when they are associated with hypertension. Adding to this, bifurcated arteries have blockages at the bifurcation and most of the studies are based on coronary and carotid arteries. Besides, the major risk is associated with a combination of PAD, diabetes and further complication. Common femoral artery has small diameter vessels in diabetic patients and women. So, punctures around the femoral head are described as the ideal puncture site [25]. As smoking adds more issues to the existing problems in the femoral artery, flowmediated vasodilation method can be induced into the artery through exercise. Hence smoking could be neglected in the present study [26]. Furthermore, anemia is another serious problem in patients with acutemyocardial infarction that causes an increase in mortality [27]. Pre and post stent analysis was conducted at the bifurcation of intracranial artery. The velocity variations and the wall shear stresses are studied and compared to observe the effect of stent placement on flow dynamics at the bifurcation [7]. Hence, various medical conditions play a major role in atherosclerosis affected arteries. Thus in the present work, peak wall shear stresses and velocities are computed from the PAD and co-morbid affected bifurcated blocked femoral artery models (Figures 1-3).
Computational analysis
A two-dimensional femoral artery is modeled with seventy percent blockage at the bifurcation (Figure 4). Another model is designed is assumed with a stent at the bifurcated artery and a blockage downstream of the stented artery (Figure 5). To compare the stented and blocked arteries, a two-dimensional artery is modeled with no blockage in artery and blockage downstream the bifurcated region. To obtain more precise values during computational analysis, the model is meshed with uniform interval size and high mesh density in ANSYS FLUENT 12.1 version. Blood is usually a non-Newtonian fluid flowing through the artery. However, blood behaves as Newtonian fluid at high strain rate [28-36] and the density of blood is assumed 1050 kg/m3. The cell zone inside the artery is selected as blood and a steady state blood flow is assumed in the analysis. Pressure inlet boundary condition is defined with mean arterial pressure values obtained from the systolic and diastolic blood pressures. The blood flow in the artery is assumed to be affected by prehypertension, hypertension stage 1 and 2.
In addition to hypertension, analysis is conducted in artery with diabetes mellitus and anemia affected blood. A clinical study has determined the blood viscosity range of diabetic patients with and without hypertension (Table 2). The present analysis is conducted using the data range provided in Table 3 with comorbidities. Diabetes and anemia can also be defined using hematocrit count (Hct) (Tables 4 and 5).
| Viscosity Factor Blood Viscosity (cP) | Diabetic with Hypertension Viscosity Factor Blood Viscosity (cP) | Diabetic without Hypertension Viscosity Factor Blood Viscosity (cP) |
|---|---|---|
| Men | 9.2 ± 4.8 | 5.5 ± 0.9 |
| Women | 6.1 ± 1.9 | 5.9 ± 1.3 |
Table 2: Experimental Data [28].
| Hypertension (MAP) Inlet | Normal Artery (Viscosity) Pa.s Material Property | Anemia (Viscosity) Pa.s Material Property | Diabetes Mellitus (viscosity) Pa.s Material Property |
|---|---|---|---|
| 93 | 0.0035 | 0.00257 | 0.00802 |
| 100 | 0.0035 | 0.00257 | 0.00802 |
| 110 | 0.0035 | 0.00257 | 0.00802 |
| 120 | 0.0035 | 0.00257 | 0.00802 |
| 130 | 0.0035 | 0.00257 | 0.00802 |
Table 3: Material Properties in Present Work.
| Health Condition | Hemotocrit counts (Hct) | Viscosity (Pa.s) |
|---|---|---|
| Anemia | 25% | 0.00257 |
| Normal | 45% | 0.00345 |
| Diabetes Mellitus | 65% | 0.00802 |
Table 4: Relation Between Viscosity and Hemotocrit Count [37].
| Artery | Length (mm) | Radius (mm) |
|---|---|---|
| Femoral Artery | 130 | 6 |
| Profunda Femoris | 100 | 6 |
Table 5: Geometry of Femoral Artery [38].
Governing equations
The flow inside the femoral artery is considered to be incompressible and blood is a non-Newtonian fluid with constant fluid properties. The continuity and Navier-Stokes equation for a two-dimensional cylindrical coordinates (r, z) can be written in a differential conservation form which is shown below.
Continuity Equation for three-dimensional arterial flow is given by:
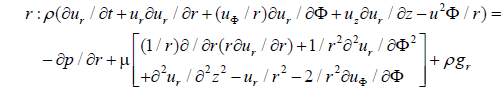 (1)
(1)
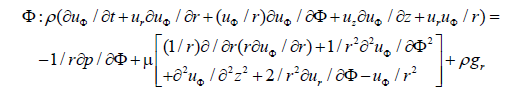 (2)
(2)
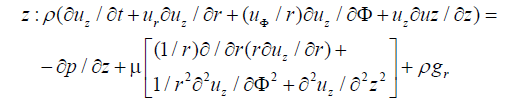 (3)
(3)
where u is the velocity with which blood flows, t is the time taken, ρ is the density of the blood, (r, φ and z) are direction of blood flow and p is the pressure.
Applying equations 1, 2 and 3 to a two-dimensional arterial flow with blockage downstream of stented region the equation is reduced to
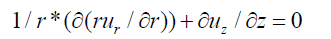 (4)
(4)
In the above equations 1, 2 and 3, for axisymmetric flow there is no tangential velocity (uφ=0) and gravity effects are neglected.
Momentum equation in r-direction is given by
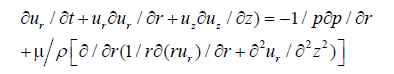 (5)
(5)
Similarly, momentum equation in z-direction is given by
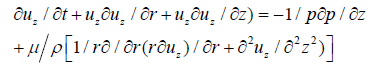 (6)
(6)
Boundary conditions
The boundary conditions used in the present analysis are:
Steady flow condition: At inlet to the artery, blood flows at a constant pressure and its equation is given by
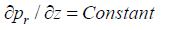 (7)
(7)
where p is the mean arterial pressure at different hypertension stages.
At the outlet of the artery, the pressure with which blood flows is given by:
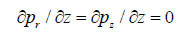 (8)
(8)
At the wall surface of the artery:
No slip flow condition is assumed: uz=0
A two-dimensional femoral artery is analyzed when subjected to comorbidities with blockage at the bifurcation. A steady state mean arterial pressure inlet boundary condition is defined to obtain the velocity and wall shear stress at the blockage. Mean arterial pressure is defined as the average arterial pressure during a single cardiac cycle.
It can be determined from the measurements of systolic and diastolic pressures at various heart rates [29].
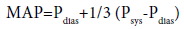
Mean arterial pressure varies with respect to prehypertension, hypertension stage 1 and hypertension stage 2 (Table 6).
| Blood Pressure Stages | Systolic Pressure (mm of Hg) |
Diastolic Pressure (mm of Hg) |
Mean Arterial Pressure (mm of Hg) |
|---|---|---|---|
| Normal Blood Pressure | 120 | 80 | 93 |
| Pre-Hypertension | 130 | 85 | 100 |
| High blood pressure stage 1 | 140 | 95 | 110 |
| High blood pressure stage 1 | 150 | 105 | 120 |
| High blood pressure stage 2 | 160 | 115 | 130 |
Table 6: Mean Arterial Pressure Inlet at Femoral Artery.
At several hypertension stages, maximum stress at profunda femoris blockage is observed at 45 degree angle. The peak wall shear stresses in a bifurcated coronary artery at different angles ranged between 2 to 2.5 Pa at peak systole and peak diastole blood pressures [30,31]. However, this chapter presents wall shear stresses ranging between 300 to 420 Pa due to blockage present at the bifurcation (Figures 6 and 7).
Similarly, peak stress is noted in a 45 degree bifurcated femoral artery when the blood is subjected to anemia. With an increase in blood pressure, the peak stress increases in seventy percent blocked bifurcated femoral artery. At 45 degrees bifurcated femoral artery, the peak stress observed is 330 Pa and in 30 degree and 60 degree bifurcated femoral artery, it is 270 and 250 Pa respectively (Figure 8).
In contrast to anemia affected blood, peak stress is seen in a 60 degree bifurcated femoral artery when subjected to diabetes. The peak stress values in a diabetic artery at 60, 45 and 30 degree bifurcated arteries are 650 Pa, 550 Pa and 420 Pa respectively (Figure 9). Stress in a blocked bifurcated artery with diabetes and high blood pressure is sixty percent higher than artery with high blood pressure and anemia.
In a blocked artery with high blood pressure, the velocity is obtained at 60 degrees bifurcated artery. As it is a seventy percent blocked artery, the velocity range from prehypertension to hypertension stage 2 is 6.5 m/s to 8 m/s (Figure 10). The velocity range in a 30 degree bifurcated artery showed lower results when compared to 45 degree bifurcation at varying blood pressures. Fistula also has bifurcated angles and Iordache et al. conducted an analysis where retrograde blood flow is high in 60° [32]. The variation in the velocities in these two studies is due to the blockage at the bifurcation and effect of comorbidities are considered in this chapter.
Similarly, the velocity is high for a 60 degree bifurcated artery when blood is affected by anemia. The peak velocity in a 60, 45 and 30 degree bifurcated arteries are 8.3, 7.2, 6.3 m/s respectively. The velocity at the blockage in an anemia and high blood pressure affected artery is higher than the femoral artery with a high blood pressure artery (Figure 11). The variation is between 3 to 5% and it is due to the blood viscosity.
In contrast to the stress analysis, the velocity in the blocked artery with high blood pressure and diabetes is lower than the artery with high blood pressure and anemia. The peak velocity at the profunda femoris is observed to be 2.8 m/s in a 60 degree bifurcated artery. In addition, 45 degree bifurcated artery is relatively exhibiting very close results to 60 degrees (Figure 12).
Figure 13 differentiates several combinations of comorbidities in seventy percent blocked femoral artery. Eventhough, the analysis was conducted for 30, 45, 60 degree blocked bifurcated femoral arteries at several comorbidities, more detailed study is required for 60 degree bifurcated artery. Figures 9 and 12 indicates that in a diabetic affected artery peak velocities and stresses are noted in 60 degree bifurcated artery. It is observed that the maximum velocity in a blocked artery is high in artery with high blood pressure and anemia when compared to diabetic blood. In contrast, the stress in a blocked artery is low in artery with anemia and high blood pressure when compared to diabetic artery with high blood pressure (Figure 14).
A self-expanding stent is modeled into the blocked femoral artery at the bifurcation. A few months after the stent implantation, blockage occurs downstream of the stented artery. Hence, blockage downstream of the stented region is modeld and analyzed. The blocked stented artery is subjected to several comorbidities like the high blood pressure, anemia and diabetes. To compare the wall shear stresses of the blocked stented femoral artery when subjected to comorbidities, a normal artery is modeled and analyzed with same comorbidities. In a 30 degree bifurcated femoral artery, the peak stress is seen in a diabetic artery (Figure 15).
Similarly, in a stented femoral artery, maximum stress at the stented region is observed in a diabetic artery. As the stent is self-expandable, it is implanted in the straight artery as well as at the bifurcation. Figure 16 indicates the stress at the straight stented artery with 30 degree bifurcation and it is 28% higher than the normal artery with diabetes.
In similarity to Figure 15, maximum stress on the straight femoral artery is obtained from an analysis in which the artery is affected by anemia, high blood pressure and diabetes. Among the comorbidities, diabetic affected artery is subjected to high stress when compared to anemia and hypertension (Figure 17) affected artery.
45 degrees bifurcated femoral artery is analyzed when the artery has a blockage downstream of stented region. From the analysis, peak stress at the stented femoral artery is noted in diabetic affected artery. These results are same as the results of 30 degree bifurcated arteries. This is because the analyzed region is straight artery where bifurcation is not included. The bifurcation begins at the profunda femoris and hence the effect of angle can be observed only in the bifurcated stented region. In Figures 16 and 18, the stented artery is affected with blockage downstream of the bifurcated stented region.
Figures 19 and 20 indicate the maximum stress in the 45 degrees partially blocked bifurcated artery without and with stent implantation. The maximum stress is seen in diabetic artery in both the cases. However, the bifurcated artery with stent shows low wall shear stress when compared to the bifurcated artery with blockage downstream. This indicates that the stent implantation has reduced the wall shear stresses. In a previous study a stented coronary artery was analyzed and the maximum stress observed at the stented region is recorded to be greater than 2.3 Pa [33]. But, the present study exhibited a maximum shear stress of 12 Pa in the bifurcated stented region of femoral artery.
An increase in the stress values at the stented region is perceived in this chapter because the artery is affected with high blood pressure, anemia and diabetes.
Similarly, in a 60 degree bifurcated artery, maximum stress is obtained at the bifurcation with and without stent implantation (Figures 21 and 22). The stress is high in a diabetic patient in both the cases. However, the stress in stented artery is lower than the bifurcated artery with partial blockage downstream.
Peak stress is recorded in the blockage downstream of bifurcated artery with and without stent implantation. Figure 23 indicates that the stress at the blockage in an artery without stent is high at a 30 degree bifurcation. In contrast, 60 degrees bifurcated arteries showed peak stress in the blockage in stented femoral artery (Figure 24). The comorbidity existing in both stented and unstented artery is diabetes. In comparison, a two dimensional straight coronary artery was modeled with seventy percent blockage. The maximum stress noted in the analysis is 32 Pa and the maximum stress observed in this chapter is 42 Pa at normal blood pressure [34]. Variation in the two studies is due to the bifurcation angles and the analysis is conducted in this chapter is in femoral arteries with a different geometry (Figure 25).
Clinical aspects
Table 7 indicates the percentage difference between different combinations of angles at the bifurcation. The maximum percentage difference in terms of stress is noted between 45 and 60 degree bifurcation in a hypertension effected femoral artery. The percentage difference between angles that exhibits low stresses is observed between 60 and 30 degree bifurcation.
In case of conditions with hypertension and anemia, the maximum difference in stress values is recorded between 45 and 60 degree bifurcation (Table 8). In contrast, least difference in stress values is observed between 60 and 30 degree. In Tables 7 and 8, least differences in stresses are noted in normal blood pressure and high stresses in stage 2 high blood pressure.
| MAP | Maximum Stress at 60° (Pa) | Maximum Stress at 45° (Pa) | Maximum Stress at 30° (Pa) | % difference between stresses for 45° and 60° | % difference between stresses for 30° and 45° | % difference between stresses for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 303 | 328 | 305 | 7.62 | 7.01 | 0.66 |
| 100 | 314 | 345 | 314 | 8.99 | 8.99 | 0 |
| 110 | 329 | 369 | 331 | 10.84 | 10.30 | 0.60 |
| 120 | 345 | 393 | 349 | 12.21 | 11.20 | 1.15 |
| 130 | 359 | 415 | 365 | 13.49 | 12.05 | 1.64 |
Table 7: Stress and Angle Effects in Hypertension Effected Bifurcated Femoral Artery.
| MAP | Maximum Stress at 60° (Pa) | Maximum Stress at 45° (Pa) | Maximum Stress at 30° (Pa) | % difference between stresses for 45° and 60° | % difference between stresses for 30° and 45° | % difference between stresses for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 224 | 267 | 241 | 16.10 | 9.74 | 7.05 |
| 100 | 232 | 282 | 251 | 17.73 | 10.99 | 7.57 |
| 110 | 244 | 298 | 273 | 18.12 | 8.39 | 10.62 |
| 120 | 255 | 311 | 277 | 18.00 | 10.93 | 7.94 |
| 130 | 265 | 328 | 285 | 19.20 | 13.11 | 7.017 |
Table 8: Stress and Angle Effects in Hypertension and Anemia Effected Artery.
In contrast to the previous tables (Tables 7 and 8), 60 and 30 degree bifurcated femoral arteries when subjected to high blood pressure and diabetes show a maximum difference in stress values. Whereas the least difference in stress values are observed between 45 and 60 degree bifurcation (Table 9).
| MAP | Maximum Stress at 60° (Pa) | Maximum Stress at 45° (Pa) | Maximum Stress at 30° (Pa) | % difference between stresses for 45° and 60° | % difference between stresses for 30° and 45° | % difference between stresses for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 539 | 506 | 320 | 6.12 | 36.75 | 40.63 |
| 100 | 568 | 523 | 345 | 7.92 | 34.033 | 39.26 |
| 110 | 593 | 555 | 357 | 6.40 | 35.67 | 39.792 |
| 120 | 634 | 576 | 386 | 9.14 | 32.98 | 39.11 |
| 130 | 683 | 599 | 444 | 12.29 | 25.87 | 34.99 |
Table 9: Stress and Angle Effects in Hypertension and Diabetes Effected Artery.
On the other hand, the maximum difference in velocity in high blood pressure affected femoral artery is noted between 60 and 30 degree bifurcation (Table 10). A similar effect of maximum difference in velocity is noted between 60 and 30 degree bifurcation artery with high blood pressure and anemia and high blood pressure and diabetes (Tables 11 and 12). The least percentage difference in velocities in high blood pressure, anemia and high blood pressure affected femoral arteries are recorded between 45 and 30 degree bifurcation. In contrast, in diabetic and high blood pressure affected femoral artery, the least percentage difference in velocity is observed between 45 and 60 degree bifurcation.
| MAP | Maximum Velocity at 60° (m/s) | Maximum Velocity at 45° (m/s) | Maximum Velocity at 30° (m/s) | % difference between velocities for 45° and 60° | % difference between velocities for 30° and 45° | % difference between velocities for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 6.69 | 5.58 | 5.23 | 16.59 | 6.27 | 21.82 |
| 100 | 6.97 | 5.81 | 5.58 | 16.64 | 3.95 | 19.94 |
| 110 | 7.35 | 6.19 | 5.83 | 15.78 | 5.81 | 20.68 |
| 120 | 7.71 | 6.42 | 6.09 | 16.73 | 5.14 | 21.01 |
| 130 | 8.07 | 6.78 | 6.33 | 15.98 | 6.63 | 21.56 |
Table 10: Velocity and Angle Effects in Hypertension Effected Bifurcated Femoral Artery.
| MAP | Maximum Velocity at 60° (m/s) | Maximum Velocity at 45° (m/s) | Maximum Velocity at 30° (m/s) | % difference between velocities for 45° and 60° | % difference between velocities for 30° and 45° | % difference between velocities for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 6.91 | 5.72 | 5.49 | 17.22 | 4.02 | 20.54 |
| 100 | 7.2 | 5.99 | 5.69 | 16.80 | 5.00 | 20.97 |
| 110 | 7.58 | 6.72 | 6.07 | 11.34 | 9.67 | 19.92 |
| 120 | 7.94 | 7 | 6.17 | 11.83 | 11.85 | 22.29 |
| 130 | 8.29 | 7.34 | 6.38 | 11.45 | 13.07 | 23.03 |
Table 11: Velocity and Angle Effects in Hypertension and Anemia Effected Artery.
| MAP | Maximum Velocity at 60° (m/s) | Maximum Velocity at 45° (m/s) | Maximum Velocity at 30° (m/s) | % difference between velocities for 45° and 60° | % difference between velocities for 30° and 45° | % difference between velocities for 30° and 60° |
|---|---|---|---|---|---|---|
| 93 | 2.33 | 2.31 | 1.43 | 0.85 | 38.09 | 38.62 |
| 100 | 2.41 | 2.39 | 1.47 | 0.82 | 38.49 | 39.00 |
| 110 | 2.52 | 2.43 | 1.52 | 3.57 | 37.44 | 39.68 |
| 120 | 2.65 | 2.59 | 1.58 | 2.26 | 38.99 | 40.37 |
| 130 | 2.77 | 2.65 | 1.61 | 4.33 | 39.24 | 41.87 |
Table 12: Velocity and Angle Effects in Hypertension and Diabetes Effected Artery.
Analysis conducted in chapter 2 could reduce the patient’s effort in clinical studies. Previous studies indicate the patient’s difficulty levels during exercise rehabilitation programs, especially when they are suffering with comorbidity. Patients with low ankle brachial index and insulin required diabetic patients are not eligible for research exercise programs [35-38].