Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2015) Volume 6, Issue 6
Purpose: To measure fluctuations of intraocular pressure (IOP) during intravitreal injection.
Materials and Methods: We worked with enucleated porcine eyes. Different volumes (0.05, 0.075, 0.1, 0.2, and 0.3 ml) of balanced salt solution (BSS) were injected into the vitreous cavity, with sham injection (0) serving as a control. IOPs were measured in real time using a digital manometer connected to a 26-gauge catheter at the anterior chamber.
Results: At baseline, the mean IOP was 4.1 ± 0.3 mmHg in the anterior chamber. A transient peak of high pressure was observed when the injection needle penetrated the sclera. A volume effect created a second peak, followed by a return to baseline after the following times (s) corresponding to the following injections: 4.4 ± 2.0 (control), 169.7 ± 6.2 (0.05 ml), 587.7 ± 83.9 (0.075 ml), 1419.2 ± 132.5 (0.1 ml), 2,381.3 ± 149.7 (0.2 ml), and 1,419.2 ± 390.1 (0.3 ml).
Conclusions: Two peaks appeared during injection. The height of the second peak and the extent of delay in recovery were dependent upon the injection volume. These results provide baseline values of IOP fluctuations during intravitreal injection.
Keywords: Intraocular pressure; Intravitreal injection
Intravitreal injection is one of the fastest growing ophthalmic procedures. Recent guidelines for intravitreal injections recommend “monitoring the IOP after injection and providing therapy when an elevated IOP warrants intervention” [1-3]. Transient IOP elevation after anti-VEGF injection is considered to be related to a volume effect caused by the addition of fluid into the vitreous cavity [4]. Several studies have described the effects of IOP in patients receiving an intravitreal injection [5-7]. However, these studies were limited in that they measured IOP intermittently after the injections. Moreover, in clinical cases, intravitreal injections consist of different volumes. Therefore, the degree of IOP elevation has not yet been fully characterized, nor are the effects of this elevation known. In order to obtain the information noted above, it is necessary to continuously monitor changes in IOP fluctuation after injection. In fact, it is nearly impossible to measure IOP in real time, especially in patients.
The literature shows that results from enucleated porcine eyes do not deviate significantly from those of living human eyes, and that the general shape and scleral rigidity are considered to be similar between porcine and human eyes [8]. Grant showed that the trabecular meshwork can be functional in enucleated eyes [9]. Thus, this study recorded real time IOP changes using porcine eyes, from the moment of injection, to evaluate IOP fluctuations according to the volume injected.
This experimental study was conducted using 36 porcine eyes (n=36). The eyes were obtained within 3 h of death and were refrigerated for 12 h after enucleation. All eyes were free of corneal damage. Pins were used to firmly mount the eyes on styrofoam. Next, a 26-gauge needle was used to connect the real-time manometer to the anterior chamber via the corneal limbus. Various volumes of balanced salt solution (BSS) (0.05, 0.075, 0.1, 0.2, and 0.3 ml), as well as the sham control (0) were injected through a 30-gauge needle to verify the effect of the injection volume on the recovery times. After injection of BSS into six eyes for each volume, the mean value of each group was calculated.
Pressure measurement
In this study, continuous IOP monitoring was started prior to the injection and maintained until the IOP returned to the baseline pressure. Pressures of 0-80 mmHg were monitored. A 26-gauge needle, connected to the manometer, was located at the anterior chamber of each porcine eye through the corneal limbus. After confirming the stabilization of the monitored IOP, BSS was injected through the sclera, using a 30-gauge needle, 3.5 mm from the limbus (Figure 1). The baseline pressures were checked before the beginning of the experiment, and fluctuations in IOP during and after the injection were recorded using a real-time manometer. The recording continued until the IOP returned to the baseline pressure.
Figure 1: Schematic drawing of the experiment. The manometer was located in the anterior chamber of the porcine eye. The manometer detected changes in pressure in the anterior chamber before and after injection. This real time change was recorded through the digital pressure sensor and digital display.
Statistical analysis
The experiment was conducted using six different injection volumes: control (0), 0.05, 0.075, 0.1, 0.2, and 0.3 ml. Six porcine eyes were used for each group. Statistical analyses were performed using the one-way analysis of variance and SPSS software, version 18.0 (SPSS, Inc., Chicago, IL). A value of p<0.05 was considered statistically significant.
Before injection, the mean baseline IOP was 4.1 ± 0.3 mmHg in the anterior chamber. The baseline IOPs were 4.3 ± 0.5, 3.8 ± 0.7, 4.4 ± 1.7, 4.4 ± 1.4, 3.6 ± 1.9 and 3.9 ± 1.4 mmHg, for each of the volumes and the sham control, respectively. There was no significant IOP difference among the six groups (p=0.062). The IOP peaked twice during the injection and returned to baseline after a certain amount of time. The first peak occurred at the moment of injection, and the second peak appeared following the first peak, due to a volume effect.
The first peak of IOP appeared when the sclera was penetrated and attained up to 40.4 mmHg. The second peak tended to be proportional to each injection volume; 8.6 ± 1.9 (0.05 ml), 8.8 ± 3.6 (0.075 ml), 9.9 ± 3.7 (0.1 ml), 10.3 ± 1.4 (0.2 ml), and 11.2 ± 1.3 (0.3 ml). The second peak in IOP was due to the injection volume (p<0.05). However, the first peak did not show a specific pattern. A detailed comparison of the pressures obtained from each injection volume is presented in Table 1. After the second peak, the IOP decreased to the baseline in a certain amount of time.
| Fluctuation of IOP after injection (1st and 2nd peak) | |||||
|---|---|---|---|---|---|
| Baseline | 1st peak | 2nd peak | Difference between baseline and 2nd peak* | ||
| Injection volume (ml) | 0 | 3.9 ± 1.4 | 13.5 ± 6.3 | 6.5 ± 2.3 | 2.6 ± 0.9 |
| 0.05 | 4.3 ± 0.5 | 16.4 ± 3.5 | 8.6 ± 1.9 | 4.2 ± 1.4 | |
| 0.075 | 3.8 ± 0.7 | 19.5 ± 12.0 | 8.8 ± 3.6 | 5.0 ± 2.9 | |
| 0.1 | 4.4 ± 1.7 | 22.7 ± 14.9 | 9.9 ± 3.7 | 5.5 ± 2.0 | |
| 0.2 | 4.4 ± 1.4 | 18.6 ± 9.4 | 10.3 ± 1.4 | 5.8 ± 0.7 | |
| 0.3 | 3.6 ± 1.9 | 25.2 ± 7.2 | 11.2 ± 1.3 | 7.6 ± 0.4 | |
Values are presented as mean ± SD. IOP=Intraocular pressure *Difference between baseline and the 2nd peak showed statistically significant differences between the groups (p<0.0,one-way analysis of variance).
Table 1: Fluctuations in IOP during injection (1st and 2nd peak) according to the injection volume.
The times (in seconds) between first and second peak were 2.7 1.0 (0.05 ml), 3.3 ± 2.4 (0.075 ml), 3.5 ± 1.4 (0.1 ml), 6.8 ± 2.2 (0.2 ml), and 7.3 ± 2.4 (0.3 ml). The times of each group tended to increase according to more injection volume. The differences in times between first and second peak were statistically significantly different (p=0.000).
The recovery time to baseline IOP was measured from the moment of injection (the first peak) to the baseline IOP. The recovery times (in seconds) were 4.4 ± 2.0 (control), 169.7 ± 6.2 (0.05 ml), 587.7 ± 83.9 (0.075 ml), 419.2 ± 132.5 (0.1 ml), 2,381.3 ± 149.7 (0.2 ml), and 4,082.1 ± 390.1 (0.3 ml) (Table 2). The recovery times of each group were different and proportional to the injection volume. However, the standard deviation tended to increase according to the volume of injection. The differences in recovery times were statistically significantly different (p<0.001). The data are presented in Table 2.
| Recovery time to the baseline IOP after injection (seconds) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Injection volume(ml) | 1st eye of the group | 2nd eye of the group | 3rd eye of the group | 4th eye of the group | 5th eye of the group | 6th eye of the group | Mean ± SD* | |
| 0 | 7.1 | 5.6 | 3.8 | 2.5 | 2.1 | 5.6 | 4.4 ± 2.0 | |
| 0.05 | 165.4 | 179.0 | 168.0 | 161.3 | 172.2 | 170.7 | 169.7 ± 6.2 | |
| 0.075 | 670.8 | 681.1 | 456.0 | 603.8 | 550.1 | 564.2 | 587.7 ± 83.9 | |
| 0.1 | 1421.8 | 1410.6 | 1319.0 | 1676.2 | 1331.8 | 1356.0 | 1419.2 ± 132.5 | |
| 0.2 | 2348.3 | 2574.5 | 2546.0 | 2261.6 | 2353.3 | 2204.3 | 2381.3 ± 149.7 | |
| 0.3 | 4673.9 | 3955.0 | 4161.7 | 3893.3 | 4284.6 | 3523.8 | 4082.1 ± 390.1 | |
*Mean recovery time showed statistically significant differences between groups (p<0.00,one-way analysis of variance).
Table 2: Recovery time to the baseline IOP after injection according to the injection volume.
In addition, the IOP fluctuations exhibited peaks and valleys; the first peak, the first valley, and the second peak occurred in sequence (Figure 2). The time from the first peak to the second peak tended to be proportional to the injection volume. However, this result was not statistically significant. Portable biomicroscopic examination showed that there was no leakage through the puncture site.
Figure 2: Fluctuation of intraocular pressure during injection of 0.3 ml of balanced salt solution. Two peaks appeared at the moment of injection of balanced salt solution. The first peak was attributable to the injection itself, but the second peak was due to a pure volume effect of 0.3 ml. This volume effect decreased and the IOP returned to baseline with time.
Intravitreal injection is rapidly gaining popularity. Rare ocular adverse events occur after intravitreal injection, including intraocular inflammation, retinal tears, vitreous hemorrhage, and endophthalmitis [10,11]. However, elevated IOP is a frequent complication of intravitreal injection [5]. Thus, it is important to monitor the IOP and to provide therapy when the elevated IOP warrants intervention after injection. However, the best time to monitor the IOP is debatable, because it is difficult to measure exact fluctuations of IOP and the recovery times back to baseline. Although many studies have reported IOP elevation after intravitreal injection [1,4,8,12,13], continuous IOP changes after intravitreal injection have not been studied. A study by Kotliar et al. (2007) evaluated the effect of intravitreal injection and volume changes on IOP, using a biomechanical model. They measured the first IOP before the injection and the second IOP immediately after the injection using a Schiøtz tonometer [13]. However, this article was limited because it could not monitor the IOP at the moment of injection. Our study provided more complete data in that we measured the real time IOP continuously, as depicted in Figure 2.
Figure 2 shows two peaks of pressure. The highest IOP was measured at the moment of perforating the sclera with a 30-gauge needle. The second IOP was measured at the moment of injection of the BSS into the vitreous cavity. The first peak is due to scleral resistance; however, it can be affected by many factors such as injection technique [12] and ocular rigidity [14]. Considering these variations, the injection volume and the height of the peaks are not exactly related, but the value of the second peak tended to be proportional to the injection volume.
The IOP elevation at the moment of injection was higher than expected, but this recovered quickly. In a clinical setting, the IOP can be elevated acutely during injection, which can damage the eye, especially eyes of patients with preexisting glaucoma [15]. In a study of sustained ocular hypertension after intravitreal anti-VEGF injection, it was noted that patients with preexisting glaucoma had a higher percentage of IOP elevation compared with those without preexisting glaucoma [16]. Thus, it is important to attempt to lower the primary peak during injection. The second peak shows the volume effect according to the volume injected. Also, the IOP recovered to the baseline after a certain amount of time. A previous study predicted that a higher pressure peak would appear after injecting more than 0.05 ml [3], but the exact relationship between the height of the peak and the injection volume was not shown. According to our results, the IOP monitoring times until the IOP returned to baseline were 2.8 minutes for the 0.05 ml injection, 9.8 minutes for the 0.075 ml injection, 23.7 minutes for the 0.1 ml injection, 39.7 minutes for the 0.2 ml injection, and 68.0 minutes for the 0.3 ml injection (Figure 3).
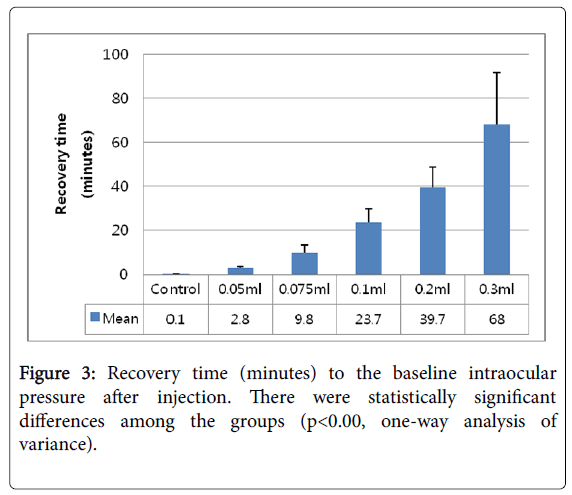
Figure 3: Recovery time (minutes) to the baseline intraocular pressure after injection. There were statistically significant differences among the groups (p<0.00, one-way analysis of variance).
Unlike previous studies, we did not measure IOP immediately after injection [6]. Thus, we did not evaluate the precise effects of injection-induced IOP elevation. In addition, it remains controversial whether anterior chamber paracentesis is essential after injection [6,13,17-19]. The present study indicates that such paracentesis is not in fact required, because IOP decreases with time after an initial steep rise.
This study had some limitations. First, we used porcine eyes. These eyes do not secrete aqueous humor, and the volume of the vitreous cavity is larger than that of the human eye [20]. Also, the baseline IOP is lower than that of the human eye. Therefore, we would expect that the IOP would increase less, and the recovery time would differ, compared to human data. Thus, our results may not exactly reflect the response of the living human eye. For example, we found that it took approximately 2.8 min for the IOP to return to the baseline level after injection of 0.05 ml. However, in human eyes, the IOP rarely returns to baseline by 10 min after injection of 0.05 ml [21].
However, previous studies found no difference in the scleral rigidity of living and enucleated human eyes from 8-57 hours postmortem [22,23], and the scleral rigidities of porcine and human eyes are similar [8]. Furthermore, Grant et al. showed that the trabecular meshwork is functional in enucleated eyes [9]. The IOP is determined by aqueous outflow, according to the Goldmann equation. Therefore, the addition of fluid volume via intravitreal injection increases the IOP, which is normalized by increased aqueous outflow [24]. We used fresh porcine eyes, all of which were enucleated within 12 h. Therefore, our results provide a basic understanding of IOP fluctuations in response to intraocular injection, although the absolute values may not be the same as those of humans.
Another limitation of our study involves well-known differences in IOP elevation resulting from injection of different drugs. Intravitreal steroids, especially, trigger a sustained rise in IOP; this develops in approximately 38.3% of eyes injected with intravitreal triamcinolone acetonide [2,5,17]. Anti-VEGF agents may exert pharmacological effects on the trabecular meshwork that could explain the sustained IOP increases [24]. Lastly, there was no leakage at the injection site when using a 26-gauge needle for IOP measurement. In clinical settings, however, leakage and vitreous incarceration at the injection site can occur [25].
Nevertheless, this study has several strengths. First, to our knowledge, this is the first study to measure real time continuous fluctuations of IOP during and after injections. This study helps to understand the degree of IOP elevation and the fluctuations of IOP during injections. Second, we measured the actual pressure with a manometer during and after the injection, and described a relationship between this volume and increases in the IOP. Moreover, it has been shown that there are two types of IOP elevations during injection. The first peak is caused by scleral rigidity and the second peak is caused by the injection volume itself. Therefore, patients with glaucoma, who may not be able to tolerate fluctuations of IOP, should be carefully managed.
In conclusion, there were two peaks during the injection process. Greater injection volumes corresponded to higher peaks, but the IOP returned to baseline with time. These experimental results provide basic data on IOP fluctuations during intravitreal injection.
We declare that no conflicts of interest in terms of this study are in play.
This work was supported by Soonchunhyang University Research Fund.