Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2013) Volume 4, Issue 2
Betamethasone sodium phosphate is a potent glucocorticoid with anti-inflammatory activity and can be used in treatment of macular edema. The aim of this work is to formulate and investigate mucoadhesive chitosan-sodium alginate nanoparticles as new vehicle for the prolonged topical ophthalmic delivery of betamethasone sodium phosphate. Ionotropic gelation method was used to produce betamethasone loaded chitosan alginate nanoreservoir system. The effect of changing different formulation parameters (pH of chitosan solution, sodium alginate concentration, calcium chloride concentration, chitosan concentration, drug concentration and the addition of tween 80) on the physicochemical properties and in vitro release of the drug loaded nanoparticles was studied. The mean particle size ranged from 16.8 to 692 nm and the zeta potential generally ranged from +18.49 to +29.83 mV depending on the formulation conditions. The highest encapsulating efficiency obtained was 64%. In vitro release studies showed an initial burst release of the drug followed by slow sustained release over 24, 48 or 72 hours depending on the formulation parameters. The in vivo studies carried out for two selected formulations showed the release of 84%, 59.5% of the drug over 12 hours for both F3C and F12 respectively. The results of physicochemical properties of F3C and F12 upon storage showed good stability at both 25°C and 40°C as the drug content was within the accepted range, the pH was (5–7) and the mean particle size for both formulations over the three months was still interesting for ophthalmic application. The results of this study suggest that chitosan alginate nanoparticles would be a promising system for the sustained release delivery of betamethasone sodium phosphate to the posterior segment of the eye.
Keywords: Chitosan; Nanoparticles; Betamethasone sodium phosphate; Ophthalmic delivery
Age-related macular degeneration and diabetic retinopathies occurring in the posterior region of the eye are the leading causes of blindness among elderly [1]. Treatments for these diseases currently include laser photocoagulation [2,3] and photodynamic therapy. However, aforementioned methods help in eliminating only the existing neovasculartization but do not treat the cause of the disease resulting in reoccurrence. Recently, corticosteroids are being used to treat posterior ocular diseases due to their angiostatic and antipermeable properties [4]. Corticosteroids show their effect by binding to the steroid receptors present in the cells. They then act by either induction or repression of the target genes and inhibit inflammatory symptoms like edema and vascular permeability [5].
Most ocular diseases are treated with topical application of drug solutions administered as eye drops. However, conventional eye drops are considered to have poor ocular bioavailability (typically less than 5% of the applied dose reaches the intraocular tissues) due to pre-ocular loss factors that include rapid tear turnover, transient residence time in the cul-de-sac and relative impermeability of the drug to corneal epilthelial membrane [6,7]. The poor ocular bioavailability imparts the need of frequent doses of concentrated drug solutions or suspensions. Ocular bioavailability can be enhanced by prolonging the pre-corneal residence time and increasing the corneal drug penetration. Due to the poor bioavailability, the use of conventional eye drops is limited to the diseases of anterior segment [8]. Various ocular drug delivery systems such as ocular inserts [9], injectable therapies [10,11], bioadhesives [12], liposomes [13], nanoparticles and nanocapsules have been investigated to improve ocular absorption and for delivery to posterior segment of the eye to treat diseases such as macular edema. Nanoparticulate systems provide enhanced absorption due to the slower elimination rate of these particles. They are also considered to be more comfortable preparations than the normal eye drops as the small particles are more tolerated by the patients than larger ones [14]. Biodegradable polymeric nanoparticles have been investigated in the pharmaceutical industry as promising ophthalmic drug delivery systems such as chitosan (CS) and alginate (ALG). Alginate has been reported to be mucoadhesive, biodegradable, and biocompatible and has potential for numerous pharmaceutical and biomedical applications such as drug delivery system and cell encapsulation [15,16]. Ionic gelation has been used to prepare alginate nanoparticles on two steps. This is done by the addition of calcium ions to form a pre-gel, followed by the addition of an aqueous polycationic solution such as chitosan solution to make a polyelectrolyte complex coating. Chitosan, a linear polysaccharide consisting of glucosamine and N-acetylglucosamine units, is biocompatible, biodegradable, and nontoxic in the application of ocular delivery of drugs [17]. Alginate–Chitosan polyionic complexes form through ionic gelation via inter-actions between the carboxyl groups of alginate and the amine groups of chitosan. The complex protects the encapsulant, has biocompatible and biodegradable characteristics, and limits the release of encapsulated drug more effectively than either alginate or chitosan alone [18]. Furthermore, considering the fact that the cornea and conjunctiva have a negative charge, it was proposed that the use of mucoadhesive polymers like chitosan, which may interact intimately with these extraocular structures, would increase the concentration and residence time of the associated drug [19].
In this study, Chitosan-Alginate nanoparticles were prepared by ionotropic gelation and were loaded with betamethasone sodium phosphate (BSP) to investigate the ability of this nanoparticulate system to deliver the drug to the posterior region of the eye, namely vitreous humor in order to localize the drug release in this region for inhibition of inflammatory symptoms like edema and vascular permeability.
Low molecular weight Chitosan (purity>90%, cps viscosity 50-300) was purchased from Bio Basic INC., Toronto, Canada. Betamethasone sodium phosphate was purchased from Upjohn, USA. Sodium Alginate was obtained from Sigma, Egypt. Calcium chloride, hydrochloric acid and lactic acid were purchase from Al Nassr Co., Egypt. Tween 80 was purchased from Corneal, France.
Preparation of chitosan-alginate nanoparticles
Both sodium alginate and chitosan solutions were prepared by dissolving the polymers in distilled water. The pH of sodium alginate solutions was adjusted to (5.4 ± 0.2) using hydrochloric acid. The chitosan solutions were prepared by dissolving the desired amount of chitosan in 1% lactic acid solution to yield the desired concentrations. The pH of the chitosan solutions was adjusted to (5.2 ± 0.2) using 1 M sodium hydroxide. Calcium chloride solutions were also prepared by dissolving the required amount of calcium chloride in distilled water to give the desired concentrations. The method used to prepare the nanoparticles is known as ionotropic-gelation. It is a two step method adapted from Rajaonarivony’s method of preparing alginate–poly-Llysine nanoparticles [20]. The first step in the nanoparticles preparation is the formation of calcium alginate pre-gel. Aqueous calcium chloride was added to 10 ml sodium alginate solution while stirring at stirring speed 400 rpm. The second step was the addition of 4 ml of chitosan solution to the resultant calcium alginate pre-gel with continuous stirring. The resultant opalescent suspension was equilibrated overnight at room temperature to allow nanoparticles to form uniform particles. The effect of individual experimental parameters (stirring times, volume of calcium chloride solution, calcium chloride and sodium alginate concentrations) on the shape and size of the nanoparticles was studied while all other variables were kept constant. By observing the shape and size of the nanoparticles under the light microscope, the regular shape was obtained using 6 ml of 0.5% calcium chloride, 10 ml of 0.5% (w/v) sodium alginate (pH 5.4), 0.08% w/v chitosan (pH 5.2) and stirring times 1.5 hr after the addition of calcium chloride solution and 1.5 hr after the addition of chitosan solution. These conditions were used when loading the nanoparticles with the drug.
Preparation of betamethasone sodium phosphate loaded chitosan alginate nanoparticles
The required amounts of BSP were dissolved in the sodium alginate solutions to give the desired concentrations (0.2, 0.4, 0.6 and 0.8%) then the same procedure was adapted as under placebo.
Formulation parameters were also varied to study their effect on the encapsulating efficiency, particle size, zeta potential and in vitro release of the formulations. These parameters included: the pH of chitosan solutions, concentrations of chitosan solutions, further changes in the sodium alginate and calcium chloride solutions, and the BSP concentrations. Furthermore, tween 80 which is a nonionic surfactant was added as a solubilizing agent to prevent the agglomeration of nanoparticles. Different concentrations of tween 80 (0.5, 1 and 1.5%) were used to study its effect on the physicochemical properties and the in vitro release. Table 1 shows the composition of the formulations prepared. All the formulations were prepared by adding 6 ml of calcium chloride solution to 10 ml of sodium alginate solution containing the drug and stirred at 400 rpm for 1.5 hrs. Then 4 ml of the chitosan solution was added with continuous stirring for another 1.5 hrs. The resultant opalescent suspension was equilibrated overnight to allow nanoparticles to form uniform particles.
| Preparation no. | Na Alginate concentration % | Tween 80 concentration % | BSP concentration % | CaCl2 concentration % | Chitosan concentration % | Chitosan pH |
|---|---|---|---|---|---|---|
| F1 | 0.50 | ……. | 0.20 | 0.50 | 0.08 | 4.2 |
| F2 | 0.50 | …….. | 0.20 | 0.50 | 0.08 | 5.2 |
| F3 | 0.50 | …….. | 0.20 | 0.50 | 0.08 | 6.2 |
| F4 | 0.50 | …….. | 0.20 | 0.50 | 0.08 | 7 |
| F5 | 0.75 | …….. | 0.20 | 0.50 | 0.08 | 6.2 |
| F6 | 1.00 | …….. | 0.20 | 0.50 | 0.08 | 6.2 |
| F7 | 0.50 | …….. | 0.20 | 0.75 | 0.08 | 6.2 |
| F8 | 0.50 | …….. | 0.20 | 1.00 | 0.08 | 6.2 |
| F9 | 0.50 | …….. | 0.20 | 0.50 | 0.50 | 6.2 |
| F10 | 0.50 | …….. | 0.20 | 0.50 | 0.75 | 6.2 |
| F11 | 0.50 | …….. | 0.20 | 0.50 | 1.00 | 6.2 |
| F12 | 0.50 | …….. | 0.40 | 0.50 | 0.08 | 6.2 |
| F13 | 0.50 | …….. | 0.60 | 0.50 | 0.08 | 6.2 |
| F14 | 0.50 | …….. | 0.80 | 0.50 | 0.08 | 6.2 |
| F15 | 0.50 | …….. | 1.00 | 0.50 | 0.08 | 6.2 |
| F3a | 0.50 | 0.50 | 0.20 | 0.50 | 0.08 | 6.2 |
| F3b | 0.50 | 1.00 | 0.20 | 0.50 | 0.08 | 6.2 |
| F3c | 0.50 | 1.50 | 0.20 | 0.50 | 0.08 | 6.2 |
Table 1: Preparations of betamethasone sodium phosphate loaded chitosan alginate nanoparticles of different compositions.
Quantitative Analysis of betamethasone sodium phosphate
BSP was assayed using UV spectrophotometer (Jenway 6315, UK). A solution of BSP (10 μg/ml) was prepared by dissolving BSP in distilled water. The solution was scanned for UV absorption and it showed maximum absorbance at 246 nm (λmax=246 nm). A calibration curve was obtained by measuring the absorbance of different concentrations of BSP solutions.
Determination of the encapsulating efficiency of betamethasone sodium phosphate chitosan alginate nanoparticles
Encapsulating efficiency of nanoparticles of different formulations was determined by centrifugation of 10 ml of the samples (both placebo and loaded samples) at 6000 rpm for 30 min. The amount of BSP was determined in clear supernatant by UV spectrophotometry at 246 nm using supernatant of placebo nanoparticles as basic correction (blank). One milliliter of the supernatant was diluted to 100 ml and the absorbance was measured. The encapsulating efficiency was calculated using the following equation:
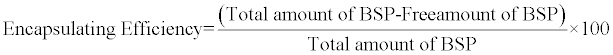
Determination of the physicochemical characteristics of betamethasone sodium phosphate nanoparticles
Morphology of chitosan-alginate nanoparticles: One drop of BSP loaded chitosan-alginate nanosuspension was added onto a copper grill and dried at room temperature. The morphology of the nanoparticles was observed using transmission electron microscopy ([Joel 100CX], Japan).
Average particle size and size distribution: The average particle size and size distribution was measured by dynamic light-scattering method (Zeta potential/particle sizer NIOCOMP 380 ZLS, USA) at 25°C with a detection angle of 90° using NUMBER-WT NIOCOMP distribution. In order to analyze, 1 ml of the nanosuspension was diluted to 10 ml with distilled water.
Zeta potential: The zeta potential of the prepared nanoparticles was determined by dynamic light-scattering method using the electric field 10 V/cm [Zeta potential/particle sizer NIOCOMP 380 ZLS, USA]. In order the measure the zeta potential of the particles, 10 ml of the sample was centrifuged and the supernatant was discarded. The precipitated nanoparticles were re-suspended in 10 ml distilled water.
pH: The pH of the prepared nanoparticles was measured using pH meter 3510 Jenway.
Viscosity: The viscosity of the selected nanoparticles was measured using Ostwald viscometer.
FT-IR analysis: FT-IR spectra were obtained to investigate if there is an interaction between chitosan/alginate and BSP. FT-IR spectra were recorded on Nicolet Avatar 380 spectrometer, USA.
Drug content
The Drug content of the prepared formulations was measured by diluting 1 ml of the formulation to 100 ml then it was analyzed spectrophotometrically to determine the actual concentration of BSP in the formulation.
In vitro release
The in vitro release of the different formulations prepared was studied using cellophane membrane (30/32). The cellophane membrane was fixed into one side of test tube opened from the two sides (diameter 0.4 cm), while 1 ml of the prepared nanoparticles was injected from the other side to come in contact with the membrane. The entire surface of the membrane was immersed in the receptor compartment containing 10 ml of distilled water in a beaker previously heated at 37°C and the receptor compartment was continuously stirred at 200 rpm using a magnetic stirrer.
At a predetermined period of time (0.25, 0.5, 1, 2, 3, 4, 5, 6, 24, 48 and 72 hr), 1 ml of the receptor compartment’s solution was withdrawn and replaced with 1 ml fresh distilled water. The withdrawn sample was analyzed for the drug content using UV spectrophotometer at 246 nm against placebo chitosan alginate nanoparticles as base of correction (blank chitosan-alginate nanoparticles were also tested for the in vitro release and a sample was withdrawn at the same time intervals in the same manner as the BSP loaded nanoparticles).
Kinetics of drug release from betamethasone sodium phosphate nanoparticles
In order to understand the kinetics of drug release, the results of in vitro drug release study of nanoparticles were fitted with various kinetic equations like zero (cumulative % drug release vs time), first order (log cumulative % drug remaining vs time), and Higuchi’s model (cumulative % drug release vs. square root of time).
The zero order kinetics describes the system where the drug release rate is independent of its concentration [21]. The equation of zero order kinetics is described as below:
C=kot (1)
Where, Ko is zero-order rate constant expressed in units of concentration/time and t is the time.
The first order kinetics describes the release from system where release rate is concentration dependent [22]. The first order equation is:
LogC=LogCo - kt / 2.303 (2)
Where, Co is the initial concentration of drug and K is first order constant.
Higuchi (1963) describes the release of drugs as a square root of time dependent process based on Fickian diffusion [23]. Higuchi’s equation is:
Q=Kt1/2 (3)
Where, K is the constant reflecting the design variables of the system.
In vitro permeation study through rabbit’s sclera
In vitro permeation through rabbit’s sclera was studied for the BSPloaded nanoparticle formulations (F12 and F3C) using the previous described technique of the in vitro release. However, instead of using cellophane membrane, rabbit’s sclera was used. Rabbits were sacrificed and the sclera was surgically removed intact from the animal’s eye socket. At the end of the experiment, the solution in the receptor compartment was examined under the microscope for the presence of nanoparticles. It was also centrifuged to precipitate the nanoparticles if they exist. This was done to investigate if the BSP nanoparticles permeate the sclera.
In vivo study of F12 and F3C
The in vivo study was carried out for both F12 and F3C. Benzalkonium chloride (0.02%) was added to each sample as preservative. Thirty two rabbits weighing 2 to 2.5 kg were used. 30 μl of the formulation or the blank (placebo nanoparticles) was applied to each eye. For each sample, 16 rabbits were used. For each time interval, the blank was instilled to one right eye while the sample was instilled to three eyes (two left eyes, one right eye). Rabbits were sacrificed after 1, 2, 3, 4, 5, 6, 12 and 24 hrs, eyes were enucleated and the vitreous humor was retrieved. Ophthalmic solutions of BSP (0.2%, 0.4%) were also prepared and studied for the in vivo permeation after 1 and 2 hrs using eight rabbits and the same procedure. Betamethasone sodium phosphate was extracted from the vitreous humor by the following procedure: the vitreous humor was mixed with 10 ml methanol and the mixture was centrifuged at 4000 rpm for 20 minutes. The supernatant was collected and the concentration of BSP was determined using UV spectrophotometer.
Stability studies
The physical stability of BSP loaded chitosan-alginate nanoparticles was evaluated after storage for 3 months under different temperature conditions. Betamethasone sodium phosphate nanoparticles (F12 and F3C) were stored in polyethylene plastic bottles with droppers and placed at 25° ± 2°C or at 40° ± 2°C away from light. Benzalkonium chloride (0.02%) was added to each sample as preservative to prevent the microbial growth during the storage period. At 1, 2 and 3 months, samples were withdrawn and tested for drug content, pH, viscosity and particle size. The encapsulating efficiency was tested after 3 months.
Preparation and characterization of betamethasone sodium phosphate nanoparticles: Physicochemical properties of betamethasone sodium phosphate nanoparticles
Examination of the prepared nanoparticles under the transmission electron microscope (TEM) appeared to be distinct, spherical particles with solid dense structure. However, the nanoparticles did not show a smooth surface but a fluffy appearance (Figure 1). The morphology of the nanoparticles was similar to the morphology of chitosan-alginate nanoparticles prepared by ionotropic gelataion obtained by previous studies [24]. Using dynamic light scattering, the particle sizes ranged from 16.8 nm to 692 nm depending on the experimental conditions used to prepare them. Table 2 shows the pH and drug content of all prepared formulations. The pH value of the prepared nanosuspensions ranged from 6 to 7.4 except F1 (pH 4.5), therefore compatible with ocular administration [25]. Drug content of various formulations was found to be uniform as it ranged from 90 to 100% according to the formulation parameters, only F10 and F11 showed a relatively low drug content (85%, 82.67%) respectively.
| Formula no | pH of formula | Drug Content (%)* |
|---|---|---|
| F1 | 4.5 | 95 |
| F2 | 6 | 98.96 |
| F3 | 6.7 | 102 |
| F4 | 7.4 | 90 |
| F5 | 6.6 | 100 |
| F6 | 6.7 | 97.50 |
| F7 | 6.7 | 102 |
| F8 | 6.7 | 98.77 |
| F9 | 7 | 102 |
| F10 | 6.8 | 85 |
| F11 | 6.8 | 82.67 |
| F12 | 7 | 91.46 |
| F13 | 7.1 | 100 |
| F14 | 7.1 | 94.2 |
| F15 | 7.1 | 93 |
| F3a | 6.5 | 100 |
| F3b | 6.5 | 100 |
| F3c | 6.6 | 99 |
*N=3
Table 2: pH values and drug content of the prepared nanoparticles.
The effect of changing different formulation parameters on the particle size, zeta potential and encapsulating efficiency
Different formulation parameters were varied to investigate their effect on the particle size, zeta potential and encapsulating efficiency.
Effect of pH variation of chitosan solution
Chitosan solutions were prepared at different pH values (4.2, 5.2, 6.2 and 7) to investigate the effect of changing the pH value on the nanoparticles size, zeta potential and encapsulating efficiency (Table 3). As these nanoparticles were formed by the ionic interaction between the chitosan and alginate, the pH value affected the particle size and encapsulating efficiency. Since the pKa of chitosan and alginate is 6.5, 3.38 respectively, when the pH of chitosan solution ranged from 5.2 to 6.2 (F2, F3), chitosan and alginate were partially ionized. The ionized molecule formed a compact polyelectrolyte complex through ionic interaction and resulted in small particle size and high zeta potential. Increasing the pH above pH 6.2 or decreasing it below pH 5.2 increased the particle size and decreased the zeta potential and encapsulating efficiency. At pH 7 (F4), the degree of ionization of alginate increased. Thus the electrostatic force between intra- and inter- alginate molecules increased. Therefore, the alginate molecules swelled and increased in size of nanoparticles, while chitosan was not completely soluble which resulted in the formation of less coated nanoparticles. This can be observed by the slight decrease in the zeta potential. Increasing the pH of chitosan solution above 5.2 increased the particle size and thus it increased the encapsulating efficiency of the chitosan-alginate nanoparticles. The opposite effect was observed by decreasing the pH from 5.2 to 4.2, the particle size significantly increased when the pH of aqueous solution was around 4. At this pH, alginate approaches its pKa values, the first of guluoronic acid around 3.7 [26], and a significant part of it starts precipitating and aggregating which may contribute to increased mean particle size and very low encapsulating efficiency since less alginate is available for nanoparticles formation. However, the zeta potential was not significantly affected in the pH range from 4.2 to 6.2. This result is in agreement with previous findings [26]. The authors found that the decrease of pH from 5.2 to 4.7 slightly decreases the mean particle size of chitosan-alginate nanoparticles but decreasing the pH to 4.2 increases the mean particle size significantly. This also comes in agreement with the results of Douglas and Tabrizian [27] who demonstrated that an alginate solution of pH 5.3 generally produces smaller particle size when combined with chitosan (pH 5.5). Additional studies were carried out and showed that within the pH range 5.1 – 5.7, the amine groups of the chitosan are protonated and the carboxyl groups of alginate are ionized, which is most important for the optimum interaction and polyionic complex formation [26,28].
| Formula no | pH of chitosan solution | pH of resulted nanoparticles | Average Particle Size (nm) | Zeta Potential (mV) | Encapsulating Efficiency (%) * |
|---|---|---|---|---|---|
| F1 | 4.2 | 4.5 | 163.2 ± 23.5 | + 22.44 | 5.82 ± 0.1 |
| F2 | 5.2 | 6 | 59 ± 8.3 | + 23.42 | 54.5 ± 0.5 |
| F3 | 6.2 | 6.7 | 99.4 ± 20.3 | + 24.21 | 64.29 ± 0.12 |
| F4 | 7 | 7.4 | 249.6 ± 44.7 | + 18.49 | 64.9 ± 0.83 |
*N=3
Table 3: Effect of pH variation of chitosan solution on the particle size, zeta potential and encapsulating efficiency of the prepared nanoparticles.
The optimum pH selected as a base for the rest of study was pH 6.2, since at this pH the nanoparticles showed an optimum particle size, zeta potential and encapsulating efficiency.
Effect of changing calcium chloride and sodium alginate concentration
Increasing the concentration of either calcium chloride or sodium alginate increased the particle size and decreased the encapsulating efficiency. This can be explained on the basis of the fact that it is the calcium chloride and sodium alginate that makes the bulk of nanoparticles matrix and less volume is available for the drug encapsulation. The results are in agreement with previous studies that showed the increase in the concentration of either the alginate or chitosan increases the particle size and decreases the encapsulating efficiency [29]. When the concentration of sodium alginate was 1% (F6) it resulted in significant increase in the particle size due to high amount of alginate forming the core of nanoparticles, and the amount of chitosan became insufficient to interact with all the carboxyl groups of alginate resulting in a negative zeta potential (Table 4).
| Formula no | Sodium alginate concentration (%) | CaCl2 concentration (%) | Average Particle Size (nm) | Zeta Potential (mV) | Encapsulating Efficiency (%)* |
|---|---|---|---|---|---|
| F3 | 0.5 | 0.5 | 99.4 ± 20.3 | + 24.21 | 64.29 ± 0.12 |
| F5 | 0.75 | 0.5 | 89.5 ± 16.1 | + 20.96 | 42.6 ± 0.87 |
| F6 | 1 | 0.5 | 229 ± 44.1 | - 20.12 | 40.32 ± 0.24 |
| F7 | 0.5 | 0.75 | 119 ± 33.29 | + 25.45 | 54.3 ± 0.67 |
| F8 | 0.5 | 1 | 170 ± 35.7 | + 22.49 | 49.5 ± 0.6 |
*N=3
Table 4: Effect of calcium chloride and sodium alginate concentrations on particle size, zeta potential and encapsulating efficiency of prepared nanoparticles.
Effect of changing chitosan concentration
Different chitosan concentrations were prepared (0.08, 0.5, 0.75 and 1%) resulting in CS/ALG ratios (1:6.25, 1:1, 1.5:1 and 2:1) respectively. The change in CS/ALG ratio affected both the particle size and encapsulating efficiency. At CS/ALG = 1:6.25, the mean diameter of the particles was the smallest (99.4 ± 20.3). This occurred because all the aminoglycoside units of chitosan neutralized the carboxyl groups of guluronic acid residues of alginate resulting in a dense structure. When the CS/ALG increased, the alginate became the limiting reactant and unreacted chitosan molecules precipitated, and formed clumps. Some chitosan molecules adhered to the surface of chitosan-alginate nanoparticles and increased the particle size with a non uniform distribution as indicated in Table 5. The increase in CS/ALG ratio slightly decreased the encapsulating efficiency (Table 5) although the particle size was increased. This is because the particle size distribution in this case was not uniform. However, the effect on the encapsulating efficiency was not significant. Some authors showed that increasing the feeding ratio between chitosan and acrylic acid causes the unreacted chitosan molecules to precipitate and adhere to the surface of chitosan poly acrylic acid nanoparticles which increases the particle size as well as the zeta potential [30].
| Formula no | Chitosan concentration (%) | CS/ALG ratio | Particle Size Distribution | Zeta Potential (mV) | Encapsulating Efficiency (%)* | |
|---|---|---|---|---|---|---|
| Average size (nm) | % | |||||
| F3 | 0.08 | 1:6.25 | 92.4 ± 20.3 | 99 | + 24.21 | 64.29 ± 0.12 |
| F9 | 0.5 | 1:1 | 50.6 ± 9.6 | 97.3 | + 25.6 | 60.15 ± 0.12 |
| 483.5 ± 84.9 | 2.7 | |||||
| F10 | 0.75 | 1.5:1 | 692.7 ± 117.8 | 100 | + 25.78 | 59.4 ± 0.75 |
| F11 | 1 | 2:1 | 97.2 ± 19.4 | 76.7 | + 25.7 | 57.19 ± 0.93 |
*N=3
Table 5: Effect of chitosan concentration on the particle size, zeta potential and encapsulating efficiency of the prepared nanoparticles.
Effect of changing betamethasone sodium phosphate concentration
To investigate the effect of BSP concentration on the particle size, zeta potential and encapsulating efficiency, different concentrations of the drug were prepared (0.2%, 0.4%, 0.6%, 0.8% and 1%). Increasing the drug concentration decreased the particle size and the encapsulating efficiency (Table 6). The decreased encapsulating efficiency with increasing drug content in the initial mixture of BSP and sodium alginate is due to the increased potential of drug for diffusion into the external solution. Previous studies also showed that increasing the dexamethasone loading into chitosan tripolyphosphate nanoparticles decreases the loading efficiency due to the diffusion of the drug into the external solution [31].
| Formula no | Drug concentration (%) | Average Particle Size (nm) | Zeta Potential (mV) | Encapsulating Efficiency (%)* |
|---|---|---|---|---|
| F3 | 0.2 | 92.4 ± 20.3 | + 24.21 | 64.29 ± 0.12 |
| F12 | 0.4 | 70.3 ± 8.2 | + 26.73 | 62 ± 0.52 |
| F13 | 0.6 | 63.6 ± 6.6 | + 27.48 | 59.4 ± 0.25 |
| F14 | 0.8 | 45.4 ± 5.7 | + 28.44 | 54.1 ± 0.66 |
| F15 | 1 | 31.3 ± 5.4 | + 29.83 | ± 0.63 |
Table 6: Effect of betamethasone sodium phosphate concentration on particle size, zeta potential and encapsulating efficiency.
Increasing the concentration of the drug decreased the particle size. This is because higher amount of drug caused the formation of more nanoparticles with smaller particle size to encapsulate the drug. Increasing the amount of drug encapsulated within the nanoparticles, lead to increasing the negative charge attracting more chitosan to the surface of the alginate nanoparticles. This increased the positive charge on the surface and the zeta potential of the chitosan alginate nanoparticles (Table 6).
Effect of adding tween 80 in different concentrations
Tween 80 was added in different concentrations (0.5%, 1% and 1.5%) to the sodium alginate solution to investigate its effect on the particle size, zeta potential and encapsulating efficiency. Tween 80 is a non ionic surfactant that is added as a solubilizing agent to prevent the agglomeration of the nanoparticles and improve physical stability. The first addition of tween 80 (0.5%) decreased the agglomeration of nanoparticles and so it decreased the particle size and the encapsulating efficiency. However, increasing the concentration of tween 80 (1 and 1.5%) increased the particle size because of the adsorption of higher amount of tween 80 which also caused the decrease in surface tension of polymers and therefore the increase in the encapsulating efficiency (Table 7). A pervious study showed that the addition of tween 80 slightly increases the nanoparticles size and the encapsulating efficiency because of the adsorption of tween 80 in the surface [32]. Since tween 80 is a kind of non ionic polymer, the more the adsorbed, the thicker the adsorbed layer and the more positive is the zeta potential. In a previous study, in which they used PLA that carries a negative charge for its end group of carboxylic acid, FITC-dextran as a non-ionic surfactant tween 80, showed that the more tween adsorbed the more positive shift in zeta potential [33].
| Formula no. | Tween 80 concentration (%) | Average Particle Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (%)* |
|---|---|---|---|---|
| F3 | 0 | 92.4 ± 20.3 | + 24.21 | 64.49 ± 0.12 |
| F3a | 0.5 | 16.8 ± 1.7 | + 19.74 | 49.48 ± 0.36 |
| F3b | 1 | 25.6 ± 2.6 | + 22 | 57.89 ± 0.32 |
| F3c | 1.5 | 61.7 ± 6.8 | + 27.8 | 60.71 ± 0.51 |
*N=3
Table 7: Effect of tween 80 concentration on the particle size, zeta potential and encapsulating efficiency of prepared nanoparticles.
Increasing the concentration of tween 80 to 1.5% increased the zeta potential and thus resulted in more physically stable nanoparticles of optimum particle size 61.7 nm and good encapsulating efficiency 60.71% as shown in Table 7.
FT-IR spectroscopy
FT-IR was adopted to characterize the potential interactions in nanoparticles. FT-IR spectrum of alginate, chitosan, betamethasone sodium phosphate and betamethasone sodium phosphate loaded chitosan-alginate nanoparticles (F12, F3C) are shown in Figures 2-6. In the spectrum of chitosan, the broad band at 3353 cm-1 corresponded to the amine and hydroxyl groups; the peak at 2867 cm-1 was caused by –OH stretching; the absorption band of carbonyl group (C=O) stretching secondary amide (amide I band) at 1625 cm-1 and the bending vibrations of N-H (N-acetylated residues, amide II band) at 1591 cm-1 [34]. The peaks at 1450 and 1382 cm-1 belong to N-H stretching of amide and ether bonds and N-H stretching (amide II band) respectively. The peaks observed at 1060 and 1022 were the secondary hydroxyl group (characteristic peak of CHOH in cyclic alcohols, C-O stretch) and primary OH (characteristic peak of –CH2-OH in primary alcohol, C-O stretch) [35]. In the spectrum of alginate, the bands around 1022 cm-1 (C-O-C stretching) are attributed to its saccharide structure. In the addition of the bands at 1593, 1402 cm-1 are assigned to asymmetric and symmetric stretching peaks of carboxylate salt groups [36]. In the IR spectrum of betamethasone sodium phosphate loaded chitosan alginate nanoparticles, the band around 3100 to 3500 cm-1 becomes broad which indicates hydrogen bonding is enhanced, the N-H bending vibration of non-acetylated 2-aminoglucose primary amines (band at 1591 cm-1) and asymmetric and symmetric –C-O stretching at (1592 and 1402 cm-1) respectively disappeared indicating that –NH3 + of chitosan has reacted with –COO- of alginate. Some of the distinct peaks corresponding to chitosan and alginate disappeared or became weaker; this might be due to multi-interactions (hydrogen bonding and electrostatic interactions) among chitosan and alginate [35]. Some characteristic absorption bands of betamethasone sodium phosphate appeared in the betamethsone loaded chitosan alginate nanoparticles, which indicate that betamethasone sodium phosphate molecule was filled in the polymeric network. However, other bands disappeared due to hydrogen bonding between the drug and the polymers. These results indicate that the carboxyl groups of alginate associate with ammonium groups of chitosan through electrostatic interactions to form polyelectrolyte complex.
In vitro release of betamethasone sodium phosphate of chitosan alginate nanoparticles
In vitro drug release studies were carried out in triplets. F1 was excluded since the encapsulating efficiency was too small. Formulations F9, F10 and F11 showed non uniformity in the particle size and low drug content (85%, 82%) for F10 and F11 respectively. Therefore, formulations F9, F10 and F11 were also excluded. For different time intervals, samples were withdrawn and the cumulative percentage of drug release was calculated. Then the release data was fitted to zero order, first order and Higuchi diffusion model.
The drug release from chitosan alginate nanoparticles followed zero order, first order or Higuchi diffusion depending on the formulation parameters. Regression value r2 suggested that the curves were fairly linear as shown in Table 8.
| Formula no | Zero order | First order | Higuchi Diffusion |
|---|---|---|---|
| r2 | r2 | r2 | |
| F2 | 0.82 | 0.9956 | 0.9686 |
| F3 | 0.9846 | 0.9983 | 0.9866 |
| F4 | 0.9245 | 0.9934 | 0.9909 |
| F5 | 0.9718 | 0.9626 | 0.9063 |
| F6 | 0.984 | 0.9942 | 0.978 |
| F7 | 0.9728 | 0.9669 | 0.8778 |
| F8 | 0.9517 | 0.8577 | 0.8352 |
| F9 | 0.8883 | 0.985 | 0.993 |
| F10 | 0.9616 | 0.9918 | 0.9929 |
| F11 | 0.8295 | 0.9855 | 0.9728 |
| F12 | 0.9776 | 0.9782 | 0.9702 |
| F13 | 0.9943 | 0.9973 | 0.9746 |
| F14 | 0.9553 | 0.9555 | 0.9724 |
| F15 | 0.9832 | 0.9948 | 0.99 |
| F3a | 0.9953 | 0.9801 | 0.9719 |
| F3b | 0.9951 | 0.9653 | 0.9623 |
| F3c | 0.9868 | 0.9915 | 0.9861 |
Table 8: Kinetics analysis of release data of betamethasone sodium phosphate from chitosan-alginate nanoparticles.
All formulations released the drug on two stages. The formulations showed an initial burst of release followed by a slow release. The initial burst of released BSP came from the free drug in the nanosuspension and from the surface of the nanoparticles. These results come with agreement to previous findings which showed a release profile of nifedipine from chitosan-alginate nanoparticles characterized by an initial burst followed by a continuous and controlled release phase. However, the release kinetics of nifedipine does not follow Fickian law of diffusion and has to be explained by non Fickian model of diffusion [24]. Other authors also studied the in vitro release of gatifloxacin from chitosan-alginate nanoparticles and showed that the drug is released from the optimized formulation over a period of 24 hr in a sustained release manner, primarily by non-Fickian diffusion [29].
Most of the formulations released almost 90-100% of the drug at different time intervals.
The controlled release of BSP from chitosan-alginate nanoparticles is mainly dependent on the encapsulating efficiency, and the degree of interaction between the amino groups of chitosan and the carboxyl groups of alginate.
Changing the pH of the formulations affected the release of BSP (Figure 7). At pH 5.2 and pH 7 (F2, F4), almost 90% of the drug was released after 24 hrs, whereas at pH 6.2, formulation (F3) released 63% of the drug after 24 hours and 100% after 48 hrs. This is due to the high encapsulating efficiency of formulation F3 and the complete ionic interaction between chitosan and alginate at pH 6.2 as alginate nanoparticles were well coated with chitosan.
Changing sodium alginate concentration also changed the release pattern of BSP (Figure 8). Formulation F6 (Na alginate 1%), 100% of the drug was released over 24 hrs whereas for F3 and F5 (0.5%, 0.75%), almost 100% of the drug was released over 48 hrs and the release pattern was not significantly different. Formulation F6 showed faster release of the drug as it had the lowest encapsulating efficiency (40.32%) compared to F3 and F5 (64%, 42% respectively) and the largest particle size with the least chitosan available for the interaction with alginate. As the zeta potential for F6 was found to be – 20 mV, which means the chitosan did not completely coat the alginate nanoparticles.
Using different concentrations of calcium chloride (0.5%, 0.75% and 1%) did not affect the in vitro release pattern of the drug. Formulation F3, F7 and F8 released 100% of the drug after 48 hrs (Figure 9).
Increasing the concentration of BSP in formulations (F12, F13, F14 and F15) and the addition of tween 80 in formulations (F3a, F3b and F3c) slowed down the release. Formulations F12, F13, F14, F15, F3a, F3b and F3c released the drug after 72 hrs, while F3 released it after 48 hrs. The addition of tween 80 or increasing the drug concentration decreased the particle size and resulted in higher surface area coated with chitosan which slowed down the release of the drug (Figures 10 and 11).
In vitro permeation study through rabbit’s sclera
The formulations that showed better physicochemical parameters with prolonged release were selected for the in vitro permeation studies. Out of the 18 formulations, only 2 formulations were selected. The selected formulations were F12 and F3C. Formulation F12 has a particle size of (70.3 ± 8.2 nm), zeta potential +26.73 mV whereas F3C has a particle size of (61.7 ± 6.8 nm) and zeta potential +27.8 mV. The two formulations were considered to be promising as they showed a prolonged release of BSP with almost 100% after 72 hours and followed first order kinetics with r2 values 0.978, 0.9915 for F12 and F3C respectively.
In the in vitro permeation study through sclera, both formulations (F12, F3C) released almost 90% of the drug within 72 hrs (Figures 12 and 13). The flux and permeability coefficient were calculated using Ficks law of diffusion. The steady state flux was calculated as shown in the equation below:
Flux = (dM/dt)/A
dM/dt: The rate of the diffusion of the drug across the sclera
A: Area available for diffusion, where A=0.5 cm2
The permeability coefficient was calculated by dividing the flux by the donor concentration (Cd) of the drug.
Permeability Coefficient = Flux/Cd
The steady state flux of F12 (0.1048 mg. cm-2.hr-1) was double the value of the flux of F3C (0.0508 mg. cm-2.hr-1). This is because the BSP concentration in F12 is double the concentration of BSP present in F3C. However, the permeability coefficient of both formulations was very close (0.0262 and 0.0254 cm. hr-1) for F12 and F3C respectively. The solution in the receptor cell for both formulations was examined after the permeation study for the presence of penetrated nanoparticles. It was examined under the microscope which confirmed the presence of nanoparticles. The solution was also centrifuged and the suspended nanoparticles were precipitated. This indicated that the nanoparticles penetrated the sclera.
In vivo study of F12 and F3C
Since both F12 and F3C showed a prolonged in vitro release and good sclera permeability, they were both tested in vivo. Benzalkonium chloride (0.02%) was added to each formulation to prevent any microbial growth. Ophthalmic solutions of betamethasone sodium phosphate (0.2% and 0.4%) were also tested in vivo.
Formulation containing 0.4% of BSP (F12) delivered 68% of the drug to the vitreous humor over 6 hrs, and the percentage was then decreased to 59.5% and 34.1% at 12 hr and 24 hr respectively. Thus the drug was still present after 24 hrs with a significant amount. On the other hand, ophthalmic solution of BSP 0.4% delivered (14.6 ± 2.6 %) of the drug after 1 hr and the drug disappeared after 2 hrs. The in vivo pattern of formulation F12 is shown in Figure 14.
Formulation containing 0.2% BSP and 1.5% tween 80 (F3C) delivered 84% of the drug to the vitreous humor over 5 hrs then the percentage started to decrease to reach 68.5% after 12 hrs and disappeared after 24 hrs. Ophthalmic solution of BSP (0.2%) delivered (8.3 ± 1.3 %) of the drug within one hour and the drug disappeared after 2 hrs. The in vivo pattern of formulation F3C is shown in Figure 15.
Formulation F12 showed a peak of 68% at 6 hrs whereas F3C showed a peak of 84% at 5 hrs. Both formulations showed sustained release pattern and good permeability of BSP compared to the BSP ophthalmic solutions.
Stability studies
The stability studies were carried out for the two formulations F12 and F3C since they showed promising results regarding, particle size, zeta potential, encapsulating efficiency, in vitro release, sclera permeation and in vivo results. Both formulations were stored at 25°C and 40°C for three months. The results of drug content, pH, viscosity, particle size and encapsulating efficiency are listed in Tables 9 and 10.
| F12 | Time (month) | Drug Content (%)* | PH* | Viscosity (Pa*s)* | Particle Size (nm)* | Encapsulating Efficiency (%)* |
|---|---|---|---|---|---|---|
| Initial | 0 | 91.46 | 7 | 8.5 | 70.3 ± 8.2 | 62.35 |
| 25°C | 1 | 91.46 | 6.9 | 8 | 73 ± 16 | _____ |
| 2 | 91.3 | 6.9 | 8 | 89 ± 16.1 | _____ | |
| 3 | 91 | 7 | 7.8 | 81.7 ± 15.2 | 60.36 | |
| 40°C | 1 | 91.46 | 6 | 7 | 75 ± 14.9 | _____ |
| 2 | 87.39 | 6 | 6.8 | 118.6 ± 15.2 | _____ | |
| 3 | 88.7 | 5.8 | 6.5 | 115.2 ± 21.8 | 51.25 |
*N=3
Table 9: Stability study of betamethasone sodium phosphate nanopartices (F12) at 25ºC and 40ºC.
| F3C | Time (month) | Drug Content (%)* | PH* | Viscosity (Pa*s)* | Particle Size (nm)* | Encapsulating efficiency (%)* |
|---|---|---|---|---|---|---|
| Initial | 0 | 98.88 | 6.6 | 7.1 | 61.7 + 6.8 | 60.71 |
| 25°C | 1 | 97 | 6.6 | 7 | 45.3 + 7 | _____ |
| 2 | 95.6 | 6.6 | 7 | 96.1 + 18.3 | _____ | |
| 3 | 96 | 6.5 | 7 | 122.5 + 21 | 60.5 | |
| 40°C | 1 | 95.8 | 5.6 | 6.5 | 29.2 + 4.8 | _____ |
| 2 | 92 | 5.6 | 6 | 23.4 + 4.6 | _____ | |
| 3 | 90.89 | 5.2 | 5.8 | 28.6 + 7.4 | 55 |
*N=3
Table 10: Stability study of betamethasone sodium phosphate nanopartices (F3C) at 25ºC and 40ºC.
For F12, there was no significant decrease in the drug content over the three months at both temperatures. Both pH and the viscosity were not changed when the formulation was stored at 25°C for three months. However, at 40°C the pH dropped from 7 to 6 and the viscosity was also decreased from 8.7 to 6.5 after three months. The particle size was slightly increased especially when stored at 40°C. The encapsulating efficiency measured after three months was almost the same at room temperature but it decreased from 62.35% to 51.25% at 40°C. When F12 was stored for three months at room temperature, there were no significant changes in the physicochemical characteristics.
For F3C, there was no significant change in the drug content, pH or the viscosity when it was stored at room temperature. At 25°C, the mean particle size increased due to the agglomeration of nanoparticles but the encapsulating efficiency was not affected. When F3C was stored at 40°C, the drug content decreased to 90.89% but this value was still within the accepted range. Both pH and viscosity also decreased. The particle size and the encapsulation efficiency were both decreased after 3 months.
The results of physicochemical properties of F3C and F12 upon storage showed good stability as the drug content was within the accepted range, the pH was close to that of tear fluid (5-7) which is compatible to ocular administration. The mean particle size for both formulations over the three months was still interesting for ophthalmic application.
I’d like to express my deep gratitude and appreciation for the National Institute of Nuclear Energy for giving me the opportunity to use the Zeta sizer and TEM to measure my samples.