Journal of Developing Drugs
Open Access
ISSN: 2329-6631
+44 1478 350008
ISSN: 2329-6631
+44 1478 350008
Research Article - (2016) Volume 0, Issue 0
Current study emphasis on development of an improved formulation of NFX HCl tablets that must be acceptable with reasonable limits of standards required for tablet. Central premise of research conducted was to evaluate and compare two available forms of Norfloxacin raw material, NFX base and another it’s acidic salt NFX HCl in tablet formulation and establish the effect of acidic and basic form of Norfloxacin. NFX base has been widely used in tablet formulation while Norfloxacin HCl is newly available form in market. After tablet preparation from each form, physical and chemical characterization was performed. Tablets formulated with Norfloxacin base complies with all specifications of physicochemical analysis whereas Norfloxacin HCl containing tablets exhibited twisted results in physical testing, increased hardness and disintegration. Thus an improved formulation was developed that could not interfere with physical as well as chemical characteristics. Result reveals that improved formulation with NFX HCl complies with standard of QC assessment.
<Keywords: Norfloxacin; Norfloxacin HCl; Hardness; Disintegration; Tablet formulation
NFX: Norfloxacin; NFX HCl: Norfloxacin Hydrochloride; DT: Disintegration Time; API: Active Pharmaceutical Ingredients; PVP K-30: Polyvinylpyrrolidone; DOE: Design of Experiment
Norfloxacin,l-ethyl-6-fluoro-1,4-di-hydro-4-oxo-7- (1-piperazinyl)-3-quinoline carboxylic acid, is a synthetic flouoroquinolones [1] having molecular weight 319.33.
It is a synthetic drug being the firstly selected drug for the treatment of diseases caused by V. cholera, Campylobacter, E. coli, Salmonella and Shigella [1]. The drug is also used for the treatment of urinary tract infections as well as gonorrhea and infection of eyes [2]. The recommended dosage is usually 400 mg twice daily. The half-life of NFX in serum and plasma is 3-4 hours and only approximately 30- 40% of an oral dose is absorbed [3]. Norfloxacin is a flouro quinolone antibacterial agent and derivative of nalidixic acid, Merck Index (2001).
Another form of same drug available as Norfloxacin hydrochloride (NFX HCl) with mol. wt. 355 Increased research strategies worldwide make easy to catch prompt attention of pharmaceutical industries towards the variety of new drug entities, drug derivatives and new available form of drug in the market. The availability of new drug form seats an important role in formulation development according to already existing form of that drug. However these new form may be cheaper or affordable but significant consideration needs to put that these affect the formulation factor as compare to its parent drug.
Currently Norfloxacin available in market in its acidic salt form i.e., Norfloxacin HCl (an acidic salt of basic drug Norfloxacin). An extensive literature survey was carried out using scientific websites to evaluate the use of new upcoming NFX HCl active raw material in replacement of NFX base in tablet formulation but there were no findings related to this search while Norfloxacin base is widely used in different tablet formulation like Gastro retentive Norfloxacin tablet [4], Norfloxacin dispersible tablet [5], Orally disintegrate Norfloxacin tablet [6] and in combination with other drugs [7-9]. Based on this, an effort was made through this investigation to develop a tablet formulation using NFX HCl and the prepared tablets were evaluated for in vitro release characteristics such as hardness, thickness, %friability, weight variation, disintegration and content uniformity.
Formulation of tablet comprises of active pharmaceutical ingredients (APIs) and hydrophilic swell able polymers excipients like PVP K-30. An immense interaction of these polymers and API take place when the system is exposed to aqueous medium, water will be absorbed and a viscous layer is formed that create hindrance in water penetration furthermore result in harder tablets. This is considered to be rate controlling step during tablet manufacturing by wet granulation and may be influence by concentration and chemical concentration of API and polymer excipients [10-16]. Excipients are not as such inert as they are usually considered to be but these play tremendous part when added to a formulation and effect on dosage form and availability of active ingredient. Quantity, nature and physicochemical characteristics of both excipients and active pharmaceutical ingredient are influential factors to describe the magnitude of this effect [17].
In the present study, nature of raw material require for formulation change from basic to acidic form. Presence of HCl in structure of parent NFX led to increase molecular weight and immense change in behavior during tablet formulation by wet granulation method. Tablets prepared from NFX hold routine practice but current formulation changes needed when Norfloxacin base is replace by Norfloxacin HCl i.e., basic to acidic form and on other hand increase in molecular weight and chemical structure of compound is also altered (Figures 1 and 2).
Observation made that formulation via Norfloxacin HCl interfere with water content during manufacturing step of wet granulation and lead to immense effect on the tablet hardness and DT. This approach let us to develop a formulation in such way that tablet produced from Norfloxacin HCl complies with specification of hardness and DT as well as other parameters of QC assessments.
Materials and Methods
Materials
Norfloxacin (china), Norfloxacin HCl (China), Lactose (China), Maize Starch (Pakistan) PVP-K30 (China), Magnesium stearate (Pakistan), Talcum powder (China).
Methods
Tablets were produced from wet granulation method. All ingredients were accurately weighed. Method for preparation includes following steps. Prepare starch paste in DI boil water with continuous stirring. Add active ingredient, PVP K30 and Lactose into blade mixer and mix for 15 minutes. Add starch paste to above step and mix for 5 minutes. Pass material through sieve of mesh 8. Put the material in tray dryer for drying at 70°C-80°C for 5 hours. Pass dried granules through sieve of mesh 12. Transfer granules into mixer. Then add magnesium stearate and talcum powder into mixer and carryout mixing for 15- 20 minutes. Finally granules will obtain. Tablets were compressed on 17 punch compression machine using 8 mm flat round punches. Total weight of tablet of Norfloxacin is 470 mg and tablet of Norfloxacin HCl is 520 mg.
Tablet thickness and diameter: Thickness and diameter of tablets were determined through vernier calliper (Model Curio FB- O498). Measurement of thickness and diameter is important to keep tablet size uniform.
Disintegration: DT apparatus was used to find out time require for opening of tablet. The model used was DT-2020 Version 1.00.
Hardness: Hardness tester is used to find out tablet hardness. This test is done to avoid chipping or breakage during handling and transportation. It is usually measured in term of kg/cm2 [6].
Friability: The instrument used is Friabilator; twenty tablets of known weight were put in Friabilator. Tablets undergo falling up and down from 6″ height [6]. After completion of 100 rotations the tablets were again weighed. %Friability was calculated from following formula.
%Friability=Initial weight-Final weight × 100/Initial weight
Uniformity of weights: In order to maintain uniformity of weight this test was carried out on random basis. Weights of 20 tablets were done and average weight is calculated. Mean and STD deviation were determined. Not more than two tablets out of 20 were deviate from the average weight.
Content uniformity: To determine the content uniformity weighed 20 tablets and crushed by motar and pestle. A quantity of powder equal to 400 mg of Norfloxacin and 444 mg of Norfloxacin HCl containing powder is dissolved in 0.1N HCl in 100 ml volumetric flask. Sample was diluted again as 5 ml in 100 ml. Absorbance was measured at 278 nm using 0.1N HCl as blank and % assay was estimated using following formula.
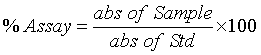
Pharmacopeial characteristics of Norfloxacin formulations (NFX base, NFX HCl and improved formulation)
NFX HCl tablets were successfully obtained by wet granulation method in replacement of Norfloxacin base. Results of physicochemical evaluations are demonstrating in Table 1 reveals that all tablets of both formulations (NFX base and NFX HCl) were reliable on physical tests, appearance (white), thickness (3.2 mm), diameter (NMT 20 mm), except hardness and DT test. NFX HCl tablets were much harder (16.9 kg) up to 2 folds than NFX base tablets and over the limits specified in BP 2005 (NMT 10 kg). Further step to hardness is to check DT which was increased in same manner, double the figure observed in NFX base tablets.
| S No | Test Applied On Tablet | Formulation With Norfloxacin Base | Formulation With Norfloxacin HCl | Improved Formulation with NorfloxacinHCl |
|---|---|---|---|---|
| 1 | Appearance | White Tablet | White Tablet | White Tablet |
| 2 | Thickness | 3.2mm | 3.2mm | 3.2mm |
| 3 | Diameter NMT 20mm |
15mm | 16.2mm | 16.2mm |
| 4 | Hardness Limit: (NMT 10kg) B.P | 8.42kg | 16.96kg | 8.36kg |
| 5 | FriabilityLimit: (NMT 1%) B.P | 0.32% | 0.19% | 0.29% |
| 6 | Avg.Wt of Tablet | 470mg ±5% | 520mg ± 5% | 520mg ±5% |
| 7 | D.T. (Nmt 15 Mints) | 10min | 23min | 11.5min |
| 8 | Weight Of Active in Tablet | 400mg / Tab | 444mg/Tab | 444mg/Tab |
| 9 | Assay | 99.2% | 98.88% | 99.1% |
Table 1: Quality Control Physicochemical Analysis highlighting Conflicted Hardness and DT Values.
Norfloxacin HCl have HCl molecule attached on 3rd position of quinoline carboxylic acid ring in structure, this lead to increase molecular weight of Norfloxacin and HCl made it acidic nature as well. Presence of HCl in structure interferes with water in wet granulation step during manufacturing and creates hindrance in penetration of water in granules, results in harder tablets than Norfloxacin base. Before developing new formulation, Assay was performed on both formulated tablets and found no effect on the availability of active ingredient in each tablet NFX base 99.2% and NFX HCl 99.1%. This approach directed studies toward excipients and their quantities used in formulation.
Quantities of ingredients and formulation development
Thus in orders to prepare tablets of Norfloxacin HCl, consideration made on quantities of binder (Maize starch paste) and disintegrate (PVP-K30) that were main attributes for hardness and DT of tablets. Starch paste used as binder and PVP-K30 actively participated in tablet opening. Quantity of formulation variables like disintegrate and binder capture significant value in design of experiment (DOE). PVP-k 30 containing tablets are faster disintegrated and less friable as compare to other disintegrate commonly used in formulation [18] hence alteration made on quantities of both starch and PVP-K30 with respect to result obtained. In new improved formulation of Norfloxacin HCl binder quantity is decreased (0.566 kg to 0.26 kg) and disintegrate quantity increase up to 2 fold (0.40 kg to 0.806 kg) to avoid harder tablets. These changes in excipients quantity produced tablets of desired hardness (NMT 10 kg) and DT (NMT 15 mints) as shown in improved formulation (Table 2). All physical and chemical characterization performed on Norfloxacin HCl improved formulation, weight of active in each tablet (444 mg), assay (99.1%), and found results satisfactory and within limits as per specifications of BP.
| S No | Formulation Ingredients | Quantities in Norfloxacin Base Tablet | Quantities in Norfloxacin HCl Tablet | Quantities in ImprovedNorfloxacinHCl Tablet |
|---|---|---|---|---|
| 1 | Norfloxacin (Active) | 10kg | 10kg | 10kg |
| 2 | Lactose (Filler) | 0.464kg | 0.464kg | 0.464kg |
| 3 | MaizeStarchpaste(Binder) | 0.566kg | 0.566kg | 0.260kg |
| 4 | PVP K30(Disintegrate) | 0.400kg | 0.400kg | 0.806kg |
| 5 | Magnesium Stearate(Lubricant) | 0.24kg | 0.24kg | 0.24kg |
| 6 | Talcum Powder(Glidant) | 0.08kg | 0.08kg | 0.08kg |
Table 2: Development in Improved Norfloxacin HClFormulated Tablets Highlighting Disintegrate and Binder Quantities.
Norfloxacin HCl tablets were manufactured successfully by wet granulation method by replacing Norfloxacin base. Increased molecular weight, changed in chemical structure and acidic form of Norfloxacin HCl did not interfered in manufacturing steps in developed formulation. Further, physicochemical parameters of improved formulation are in complete accordance with the required limits of BP.