Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Case Report - (2014) Volume 4, Issue 4
Cimetidine is a histamine H2-receptor antagonist that inhibits stomach acid production. It is largely used in the treatment of heartburn and peptic ulcers. One of the promising methods to improve the solubility and bioavailability of an active pharmaceutical ingredient is the amorphous phase which will have high free energy than that of the crystalline phase. The relaxation dynamics of cimetidine above the glass transition temperature has been studied by Broadband Dielectric Spectroscopy. Dielectric measurements of amorphous cimetidine were performed after its vitrification by fast cooling from few degrees above the melting point (412.50K). The relaxation processes observed above the glass transition temperature, were labeled as alpha and characterized. The temperature dependence of the structural relaxation time can be described over the entire measured range by single Vogel-Fulcher-Tamman (VFT) equation. From the VFT fits, the glass transition temperature was estimated as 316.27 K and a fragility or steepness index as 69, showing that cimetidine is an intermediate glass former.
<Keywords: Amorphous cimetidine, Bioavailability, Broadband dielectric spectroscopy, Glass transition, Molecular mobility, Solubility
In the pharmaceutical industry, the most popular method of drug formulation is in the solid form or crystalline form and it has a definite arrangement of atoms having long range order and is highly stable. But, the solubility and bioavailability of this form is found to be low [1]. There are different methods to improve the solubility and bioavailability of an active pharmaceutical ingredient (API). Amorphous form which is one of the recognized forms of solid matter is one of the alternatives. The amorphous form is highly unstable and reverts into the crystalline form and loss in the therapeutic activity [2]. The amorphous form is having high free energy, enthalpy, specific volume and viscosity and it offers greater solubility, dissolution rate and bioavailability. But they have high molecular mobility which will reduce the shelf-life of the drugs during processing, handling and storage [3]. It is easier to make tablets from amorphous form and can avoid the use of excipients and chemicals during the formulation process of tablets [4].
Recent studies show that molecular mobility is the key factor responsible for the devitrification of the drug in the amorphous form and devitrification can also occur even below the glass transition temperature (Tg). Therefore, it is not safe to store the drug below Tg. Hence, the production of the drug in the amorphous form is very limited even though it is economical [5,6].
Different relaxation phenomena can be observed in the amorphous state due to the increase in viscosity, alpha (α)-relaxation which is found to be the slowest one and originating from the cooperative rearrangement of molecules is present above Tg. Below the glass transition temperature, different relaxation processes can be seen due to inter or intra molecular motions called the secondary relaxations [7]. Knowledge about the molecular dynamics of amorphous pharmaceuticals will be very important in the pharmaceutical industry.
Cimetidine is chosen as model drug for the current study. Cimetidine, a histamine H2-receptor antagonist that inhibits stomach acid production, is largely used in the treatment of heartburn and peptic ulcers. Cimetidine is taken orally, absorbed adequately and its bioavailability is 60-80%. But penetration in brain is poor because of its hydrophilic nature [8]. Here we want to stress the understanding of molecular dynamics above the glass transition temperature Tg. To study the molecular dynamics of supercooled cimetidine we decided to carry out Broadband Dielectric Spectroscopic (BDS) measurements. BDS is an effective tool to probe the molecular dynamics in the amorphous state in a wide frequency (f=109 to 10-2Hz) and temperature range (264°C to - 140°C).. Recently this technique has been used as an investigaive tool to study the dielectric properties of various amorphous pharmaceuticals [9,10]. Our observation might be very useful for predicting the appropriate conditions during processing, handling and storage of this API in the amorphous form.
Material
Cimetidine, a white crystalline powder CAS NO 51481-61- 9 was purchased from Sigma Aldrich (purity ≥ 98%). Cimetidine is chemically described as 2-cyano-1-methyl-3-(2-[5-methyl-1Himidazol- 4-yl) methylthio] ethyl) guanidine. Its empirical formula is C10H16 N6S and molecular weight is 252.34 gmol-1. The chemical structure is presented in Figure 1. The purchased material was used without further purification.
Broadband dielectric spectroscopy
Dielectric measurements at ambient pressure were carried out using Novo-Control GMBH alpha analyzer covering a frequency range from10-2 to 107 Hz. Temperature was controlled using nitrogen gas cryostat with temperature stability better than 0.1 K. The tested sample was placed in a measurement capacitor made of stainless steel (diameter: 30 mm, gap: 0.20 mm). Teflon is used as the spacer. Dielectric measurements of cimetidine were performed after its vitrification by fast cooling (10 K/min) from a few degrees above the melting point (Tm= 412.50K). The temperature measurements were carried out from 324.15K-368.15K in steps of 2K and from 368.15K to 373.15K in steps of 5K. The sample does not crystallize during cooling from the melting temperature. It means that cimetidine is a very good glass former and can be easily supercooled.
The dielectric loss spectra (i.e. imaginary part of dielectric permittivity plotted as a function of frequency) above the glass transition temperature Tg is shown in Figure 2a. Tg is defined as a temperature at which dielectric relaxation time τα is equal to 100 s and its value for cimetidine is 316.27 K. Dielectric loss spectra collected above Tg exhibits α-relaxation and dc-conductivity. dc-conductivity is due the translational motions of ions from the nitrile group. To get a clear picture of the α-peak, conductivity is subtracted from the total dielectric loss and the spectra above the glass transition temperature is fitted using Havriliak-Negami (HN) function along with an ionic conductivity term and presented in Figure 2b.
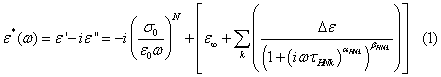
The first term denotes the conductivity effects 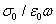 and added to the imaginary part of the fit function σ0 is the conductivity due to the translational motion of the ions, and after a fit is complete the conductivity term can be subtracted from fit function of the measured data, ε0 is the dielectric permittivity of vacuum. N is the exponent related to the ionic conductivity of the sample which is due to the mobility of ions. Second term is the HN function. Where k sums over different relaxation processes, ω is the angular frequency, τHN is the characteristic relaxation time which is related to the frequency of maximal loss fmax, Δε is the dielectric strength ,
and added to the imaginary part of the fit function σ0 is the conductivity due to the translational motion of the ions, and after a fit is complete the conductivity term can be subtracted from fit function of the measured data, ε0 is the dielectric permittivity of vacuum. N is the exponent related to the ionic conductivity of the sample which is due to the mobility of ions. Second term is the HN function. Where k sums over different relaxation processes, ω is the angular frequency, τHN is the characteristic relaxation time which is related to the frequency of maximal loss fmax, Δε is the dielectric strength ,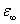 the high frequency limit of the real part
the high frequency limit of the real part 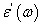 ; αHN, βHN are the shape parameters. The relaxation time τ can be calculated by using the equation given below
; αHN, βHN are the shape parameters. The relaxation time τ can be calculated by using the equation given below
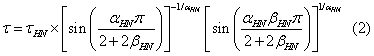
A well-pronounced α- peak moving towards the lower frequency side characterizes the increase of structural relaxation with decreasing temperature. From 360.15 K onwards the dielectric strength of α-process starts decreasing in a non-significant manner, which shows tendency for crystallization of cimetidine.
Dielectric spectra obtained after removing the conductivity contribution were used for further analysis of the main relaxation process (α-relaxation). In the supercooled region close to the glass transition, the temperature evolution of the α-relaxation time τα, very often follows the time honoured Vogel–Fulcher–Tamman (VFT) equation [11],
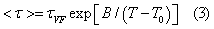
Where τvF, B, T0 are constants. The temperature T0 is often referred to the Kauzmann temperature TK, a hypothetical temperature where configurational entropy of super cooled liquid would be equal to entropy of the crystal and molecular mobility would reduce to the same level as that of the crystal (not completely be zero). For most of the glassy systems, it lies between 50 and 70K, below Tg and it is sufficient to ensure safety storage.
From VFT fit we calculated the fragility or steepness index m of cimetidine by using the equation given below:
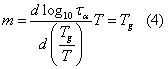
Relaxation map of cimetidine is shown in Figure 3.
Fragility is the measure of deviation from Arrhenius behavior [12]. From VFT fit the glass transition temperature (Tg) was estimated as 316.27K. This value is in close agreement with the result (Tg= 319.67K) reported by Pajula et al., in their Differential Scanning Calorimetry (DSC) experiment [13]. This relatively high value of Tg indicates the possibility of preparing oral dosage form of cimetidine in the amorphous form completely at room temperature and even at human body temperature. So, high Tg amorphous pharmaceuticals with stable glassy phase can be a better substitute than its traditional counterpart. Fragility (m) is estimated as 69, proving the system as an intermediate glass former. T0 as 238K, assuming that this corresponds to the temperature at which normally all important molecular motions stops. It is concluded that storage temperature in a freezer for cimetidine is (<238K) and should provide at least 3-5 years shelf-life stability [14]. Another method for giving stability to amorphous drugs is to raise its Tg by mixing it with high-Tg glass former such as PVP (K 29/32) [15, 16].
Finally we present the results of solubility measurements of crystalline and amorphous form of cimetidine at 37°C in methanol which is found to be the solvent of cimetidine according to Indian Pharmacopoeia [17]. During the determination of solubility the differences between both forms of examined drugs were found. The solubility of amorphous cimetidine was found to be double than that of crystalline form. This is one of the reasons for higher bioavailability of the drug.
We studied the dielectric properties of supercooled cimetidine above the glass transition temperatue. The tested sample is found to be an ionically conducting glass-former. In the whole temperature range above the glass transition a well pronounced peak indicates the α-relaxation process.
The glass transition temperature calculated from VFT fit is equal to 316.27K. The high value of Tg indicates the prepration of the drug in amorphous form at room temperature or at higher temperature for the formulation process of tablet manfacturing. A steepness or fragility index m of 69 was estimated which designates that the examined drug is an intermediate glassformer. The storage temperature for amorphous cimetidine is found to be (<238K) and this will provide at least 3-5 years shelf-life stability. Finally we present the results of solubility of crystalline and amorphous cimetidine, solubilty of amorphous form is double than that of crystalline form at 37°C.
The authors wish to thank IIT Powai, Mumbai, Product development and R&D department of AryaVaidyaSala, Kottakkal, Kerala, India, for providing experimental facility. We are profusely thankful to Dr. G Govindaraj and Mr. N.S Krishnakumar, Department of Physics, Pondicherry University for their help during the analysis. U. Sailaja acknowledges University Grants Commission, Government of India for the award of a research fellowship under the Faculty Improvement Program.