
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 3
Objective: Given the important association between frailty and Chronic Kidney Disease (CKD), we assessed the prognostic value of the Frailty Phenotype (FP) in elderly patients with advanced CKD and compared the ability of two established frailty tools to predict mortality.
Methods: Observational, prospective study with two years of follow-up. Spanish patients ≥ 65 years from the Nephrology clinic at San Carlos Hospital, with an eGFR<20 mL/min/1.73 m2, and without renal replacement therapy. All patients received functional, cognitive and nutritional assessments. Frailty was measured using established FP cut-off. Regression models were conducted to determine whether frailty was associated with greater mortality, hospital admission, and dialysis initiation. The frailty assessments included the Short Physical Performance Battery (SPPB) and gait speed.
Results: One hundred individuals (62% male; mean age 78.8 ± 7.1 years) were assessed and showed a prevalence of frailty of 44.7%. The mean follow-up was 2.1 ± 0.2 years, during which 34% started dialysis and 24% died. Frail patients had an increased adjusted risk of death (HR 5.4; 95% CI:1.859-15.866) and hospital admission (OR 3.4; 95% CI:1.247-9.534). SPPB had better predictive ability in estimating the risk of death at two years, similar to that obtained by the FP.
Conclusion: Our results support the prognostic value of the FP in the assessment of advanced CKD patients, the use of the SPPB tool in clinical practice for risk stratification of patients and the possible benefit from establishing effective interventions aimed at improving or reversing the frailty condition, thus improving the quality of life for this special population.
Elderly; Advanced chronic kidney disease; Frailty
Growing evidence shows that Chronic Kidney Disease (CKD) is associated with functional and cognitive decline in older individuals [1-6], and much consideration has been given to the contribution of major comorbidities such as heart disease, heart failure, end- stage renal disease, increased susceptibility to infections, and greater cognitive impairment.
While different studies have shown that clinical interventions can be effective in treating or preventing frailty in older people [7-12], we highlight the importance of recognition of frailty in older patients with advanced CKD and the utility of a frailty instrument useful in clinical practice as a prognostic tool to identify the patient population who will benefit most from establishing those interventions.
We conducted a longitudinal, observational, cohort study of older patients with advanced CKD to (1) estimate the prevalence of frailty using Fried’s criteria; (2) identify patient characteristics associated with frailty; (3) determine the ability of the frailty condition (according to Fried’s criteria) to predict the onset of adverse health outcomes (mortality, hospital admission, onset of dialysis); (4) compare the ability of two established frailty tools to predict mortality.
Study design and participants
This prospective, observational, cohort study aimed to determine the effect of frailty using a physical assessment among patients with advanced nephropathy. The study recruited adult patients from the Nephrology outpatient clinic at San Carlos Hospital, one of the largest hospitals in Madrid (Community of Madrid), whose kidney function was known. To be eligible for the registry, the subjects had to be ≥ 65 years old, with an estimated Glomerular Filtration Rate (eGFR) of <20 mL/min/1.73 m2, and without kidney transplant or receiving maintenance Renal Replacement Therapy (RRT) at entry, and they had to be able to be evaluated by the Geriatrics team. Candidates were prospectively enrolled between April 1, 2016 and June 31, 2017. The study was approved by the research ethics boards of San Carlos Hospital.
Participants had to sign informed consent prior to inclusion. For patients who were incapable of consenting, the legal advisor was informed and had to sign the informed consent form. The only exclusion criterion was the refusal to participate or sign the informed consent form.
Variables definition
Frailty criteria: We used the Fried frailty criteria, with the established cut-off according to Fried’s original data. Frailty and prefrailty were defined on the basis of the five dimensions described by Fried and associates. To construct the frailty phenotype variable, participants had to have valid values in at least 3 of the 5 criteria. A score of three or more classified a patient as frail, one or two classified a patient as prefrail, and zero classified a patient as robust. Participants with health-related difficulties in one or more of the five basic activities of daily living (eating, bathing, dressing, transferring, using the toilet) for at least 3 months were considered disabled.
Fried criteria were defined as follows: unintentional weight loss ≥ 4.500 kg or ≥ 5% of body weight in the last year. Weakness was indicated when the highest of two consecutive dynamometer measurements of handgrip strength using a JAMAR®digital hand dynamometer was in the lowest 20% while being adjusted for sex and Body Mass Index (BMI). Poor energy and endurance were indicated by self-reported exhaustion determined by two questions from the Center of Epidemiologic Studies Depression scale (CES-D). Slowness was indicated by having a measurement of the time taken to walk 4.6 metre that was within the lowest 20th percentile while being adjusted for sex and height. Low physical activity level was indicated by a Minnesota Leisure Time Activity Questionnaire Score within the lowest quintile for each sex.
Variable outcomes: Patients were followed for outcomes for two years, until July 2019. Outcomes included: (1) time to all-cause mortality obtained from the institution medical records and phone calls; (2) time to dialysis initiation or kidney transplantation; (3) hospitalization for medical conditions during the first year of follow-up (non-catheter-related or other surgical reasons).
We further categorized hospitalizations into number of hospitalizations, and the cause of hospitalization: cardiovascular disease, infectious disease, bleeding, stroke, and others.
Additional baseline characteristics: Baseline data were collected prospectively from patients at the Geriatrics outpatient clinic and included sociodemographic information; comorbid conditions and usual medication; anthropometric data; functional, psychological and nutritional assessment; and laboratory investigations.
Sociodemographic data included age, sex, coresidence (living alone, accompanied, institutionalized) and educational level. Educational level was categorized into 5 categories (<5, 5-10, >10-15, >15-20 and >20 years).
Comorbid conditions (hypertension, diabetes, coronary artery disease, congestive heart failure, atrial fibrillation/flutter, peripheral vascular disease, cerebrovascular disease, history of prior malignancy, chronic lung disease, lower extremity amputation, visual or hearing impairment) and cause of CKD (vascular, diabetic nephropathy, chronic interstitial nephritis; unknown; other aetiology) were recorded. Comorbidity was analyzed using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) index. High comorbidity was indicated when the CIRS-G index was equal to or greater than 2.5 points. Usual medication was also recorded and categorized into therapeutic groups. Polypharmacy was defined as regular use of at least 5 medications, and hyperpolypharmacy was considered when the number of medications was equal to or greater than 10. The use of laxatives and oral supplements was not considered. Anthropometric data included weight in kg and height in cm. Body Mass Index (BMI) was calculated in kg/m2.
All patients received functional, cognitive and nutritional assessments using the Barthel, Lawton and Functional Ambulation Classification (FAC) test; The Montreal Cognitive Assessment (MoCA); and the Mini Nutritional Assessment (MNA-SF), respectively.
Function was determined using the Barthel index, which assesses the ability to independently realize 10 Activities of Daily Living (ADLs): eating, dressing, grooming, bathing, toileting, transferring, walking and climbing stairs, and urinary and faecal continence. The Barthel index was codified as follows: total disability 0-20 points, severe disability 21–60 points, moderate disability 61–90 points, mild disability 91–99 points and independence 100 points. The Lawton index was used to evaluate 8 instrumental ADLs: managing finances, managing transportation, shopping and meal preparation, housecleaning and home maintenance, managing communication and managing medications.
Ambulation was classified with the FAC in six levels from 0 to 5 (0=inability to walk; 1=ambulation with great help from another person; 2=ambulation with light help from another person; 3=ambulation with supervision without physical contact; 4=independent ambulation on flat surfaces only, and 5=independent ambulation including stairs). Cognitive function was determined with the MoCA test, and participants were classified with a cut-off point <24 or ≥ 24. For identification of nutritional status, the MNA-SF tool was used, and patients were classified into 3 categories: well-nourished>12 points, risk of malnutrition (8-11 points) and malnourished (<7 points).
Physical function was also determined with the Short Physical Performance Battery (SPPB) which measures balance (feet together, semitandem and tandem), gait speed in m/s (normal pace 4 m, beginning in standing position) and the timed five chair stands test in seconds. Scores range from 0 (worst physical function) to 12 (best physical function).
Finally, we collected laboratory measurements (CKD-EPI equation to estimate the glomerular filtration rate using serum creatinine, haemoglobin, haematocrit, urea, creatinine, albumin, pre-albumin, calcium, phosphorus, sodium, potassium, uric acid, parathytoid hormone, vitamin D, vitamin B12, microglobulin, glycosylated haemoglobin, PCR, total cholesterol, HDL, LDL, iron, ferritin and transferrin saturation index).
For further analysis, data for falls in the previous 1 year were collected by phone call and from the medical records of the institution.
Data collection and measurements
After the informed consent forms were signed, patients were referred from the Advanced CKD Nephrology clinic to the Geriatrics outpatient clinic, for further evaluation and assessment. Information was collected prospectively through a single, face-to-face personal interview with the participant at the institution. One trained geriatrician conducted the interviews. Patients received identical baseline evaluations. The information was provided directly by the participant or by the legal advisor if the participant was unable to do so. Interviews were supplemented by measurements of performance tests, conducted on the same day as the interview by the same geriatrician.
The information on the participant’s chronic diseases, medication and cause of CKD were collected from the institutional medical records and contrasted with the patient’s responses. Data were anonymized, codified and included in a database for further analysis. Patients received follow- up with annual telephone calls and surveillance with medical records for health outcomes including hospitalizations, onset of dialysis and mortality, over two years.
For the purposes of this study, patients who were unable to attend the geriatrician interview at baseline and could not complete the performance tests for frailty assessment were interviewed by phone. Those patients were excluded from the analysis of frailty, unless they fulfilled three points on all other Fried’s criteria.
Statistical analysis
Descriptive statistics were reported as the counts and percentages for qualitative variables, the mean ± SD for normally distributed continuous variables, and the medians and Interquartile Ranges (IQR) for non-normally distributed continuous variables.
The prevalence of frailty by Fried’s criteria was reported using a cut-off of three or more. The main frailty variable was categorized into two categories (yes/no) and sociodemographic and clinical characteristics were compared between the two study groups. The association between qualitative variables was evaluated with the chi-square test or Fisher’s exact test, when more than 25% of those expected were less than 5. The comparisons of the quantitative variables that fit a normal distribution were made using Student’s t test for two independent groups. In the case of variables that did not fit a normal distribution, the comparison was made using the nonparametric Mann‒Whitney U test.
The Kaplan‒Meier method was used to estimate survival curves for the events survival time and time to dialysis. To estimate the unadjusted and adjusted effect of the presence of frailty (yes/no) with the outcome variables survival time and time to dialysis, Cox proportional hazards regression models were adjusted. The adjustment variables were those that, in the comparison between patients with and without frailty, presented a statistically significant result (p<0.10) and/or were clinically relevant. Rate ratios (Hazard Ratio; HR) are presented along with their 95% confidence intervals. For the outcome variable need for hospital admission in the first year, a logistic regression model was adjusted. To obtain the adjusted effect of frailty, the same strategy previously described was used. The proportionality assumption of the Cox model was evaluated by applying the Schoenfeld residuals-based test.
Furthermore, for the comparison of the classification ability of frailty, gait speed and SPPB tools on the mortality rate, the HR of each tool was obtained and presented using the Cox model. The discrimination ability of each one was evaluated by calculating the C statistic and this index was compared between the three tools.
All p values were two sided, and p value<0.05 indicated statistical significance. Data processing and analysis were performed using STATA version 15.1 (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
A total of 100 patients were enrolled between April 2016 and June 2017. Of the 100 patients who agreed and gave consent to participate, 9 patients could not complete the geriatric functional assessment and were assessed by phone call. Regarding the Frailty Phenotype (FP) criteria, in 94 (94%) participants, 3 or more valid Fried criteria were available to determine frailty status. In 6 cases, fewer than 3 valid criteria were available, and frailty status could not be determined. None of those 6 participants were considered for further analysis.
Participant characteristics
Among the study participants, the mean age was 78.8 ± 7.1 years, 62% were male, and 14% lived alone. The mean follow-up was 2.1 ± 0.2 years, during which 34% started dialysis (haemodialysis 80% and peritoneal dialysis 20%) and 24% died. Only one patient received a kidney transplant after the onset of haemodialysis. Table 1, presents the baseline characteristics of the complete sample and the laboratory data.
| Sociodemographic characteristics | Mean ± SD or n (%) |
|---|---|
| Age | 78.8 ± 7.1 |
| <80 years | 52 (52.0) |
| ≥ 80 years | 48 (48.0) |
| Sex | |
| Female | 38 (38.0) |
| Male | 62 (62.0) |
| Educational level | |
| <5 years | 31 (31.0) |
| 5-10 years | 24 (24.0) |
| >10-15 years | 19 (19.0) |
| >15-20 years | 19 (19.0) |
| >20 years | 7 (7.0) |
| Social cohabitation | |
| Live alone | 14 (14.0) |
| Accompanied | 86 (86.0) |
| Functional status | |
| Barthel Index | 90.5 ± 11.7 |
| ≤ 90 | 43 (43.0) |
| >90 | 57 (57.0) |
| Physical disability ADLs | 35 (35.0) |
| Lawton Index | 4 ± 2.2 |
| FAC | |
| 0 | 0 |
| 1 | 0 |
| 2 | 2 |
| 3 | 12 |
| 4 | 16 |
| 5 | 70 |
| Nutritional characteristics | |
| BMI | 27.8 ± 4.5 |
| MNA- SF | |
| Normal | 62 (62.0) |
| Risk of malnutrition | 35 (35.0) |
| Malnutrition | 3 (3.0) |
| Aetiology of CKD | |
| Diabetic nephropathy | 28 (28.0) |
| Unknown | 24 (24.0) |
| Vascular | 23 (23.0) |
| Glomerular | 9 (9.0) |
| Chronic interstitial nephritis | 4 (4.0) |
| Other causes | 12 (12.0) |
| Comorbidity | |
| CIRS-G punctuation | 14.7 ± 3.6 |
| Number of categories | 6.14 ± 1.6 |
| CIRS-G Index | 2.5 ± 0.3 |
| High comorbidity (CIRS-G Index>2.5) | 45 (45.0) |
| Arterial hypertension | 93 (93.0) |
| Diabetes mellitus type 2 | 48 (48.0) |
| Sensory deficit | 40 (40.0) |
| Neoplasm | 34 (34.0) |
| Heart failure | 29 (29.0) |
| Atrial fibrillation or atrial flutter | 23 (23.0) |
| Ischemic heart disease | 23 (23.0) |
| Vascular peripheral disease | 23 (23.0) |
| COPD | 16 (16.0) |
| Stroke | 14 (14.0) |
| Lower limb amputation | 3 (3.0) |
| Medication | |
| Number of drugs | 9.2 ± 3.3 |
| Hyperpolypharmacy | 43 (43.0) |
| Statins | 67 (67.0) |
| ARBs | 50 (50.0) |
| Diuretics | 38 (38.0) |
| Erythropoietin | 37 (37.0) |
| Insulin | 26 (26.0) |
| Oral antidiabetics | 18 (18.0) |
| Acenocoumarol | 15 (15.0) |
| Functional Performance Tests (n=91) | |
| MoCA test: | 20.47 ± 5.9 |
| < 24 | 60 (66.7) |
| ≥ 24 | 30 (33.3) |
| SPPB | 7.0 ± 2.3 |
| Gait speed | 0.69 ± 1.0 |
| ≤ 0.8 m/s | 81 (89.0) |
| >0.8 m/s | 10 (11.0) |
| Chair and stand test | 19.4 ± 7.5 |
| Laboratory data | |
| Haemoglobin (g/dL) | 11.95 ± 1.29 |
| Haematocrit (%) | 37.38 ± 5.02 |
| Leukocytes (×103/uL) | 7.066 ± 2.116 |
| Lymphocytes (×103/uL) | 1.755 ± 1.117 |
| Urea (mg/dL) | 135 ± 45.14 |
| Creatinine (mg/dL) | 3.36 ± 0.92 |
| CKD-EPI eGFR(mL/min) | 16.05 ± 4.83 |
| Sodium (mmol/L) | 139 ± 2.80 |
| Potassium (mmol/L) | 4.78 ± 0.56 |
| Calcium (mg/dL) | 8.8 ± 0.61 |
| Phosphorus (mg/dL) | 4.05 ± 0.86 |
| Albumin (g/dL) | 3.8 ± 0.40 |
| Prealbumin (mg/dL) | 28.75 ± 9.86 |
| Total proteins (g/dl) | 6.76 ± 0.61 |
| Total cholesterol (mg/dl) | 162.19 ± 40.83 |
| LDL cholesterol (mg/dl) | 89.81 ± 37.72 |
| HDL cholesterol (mg/dl) | 51.45 ± 17.72 |
| Iron (ug/dl) | 64.80 ± 20.84 |
| Transferrin saturation index (%) | 20.85 ± 7.41 |
| Vitamin D (ng/ml) | 18.42 ± 8.06 |
| β2 microglobulin (mg/l) | 11.31 ± 10.13 |
| Uric acid (mg/dl) | 6.30 ± 1.47 |
| Median (Interquartile range) | |
| Ferritin (ng/dl) | 102.30 (56.50–170.96) |
| Polymerase chain reaction (mg/dl) | 0.29 (0.23–0.98) |
| Parathyroid hormone (pg/ml) | 202.00 (127.35–288.80) |
ADLs: Activities of Daily Living; FAC: Functional Ambulation Classification; BMI: Body Mass Index; MNA-SF: Mini Nutritional Assessment-Short Form; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; COPD: Chronic obstructive pulmonary disease; ARBs: renin angiotensin system blockers; MoCA: Montreal Cognitive Assessment; SPPB: Short Physical Performance Battery.
*Note: SD: Standard deviation.
Table 1: Baseline characteristics of the sample (n=100).
Prevalence and characteristics of frailty
The estimated prevalence rates of frailty was 44.7%, prefrailty and robustness were 44.7% and 10.6%, respectively. Regarding FP criteria, valid data for exhaustion, low physical activity and weight loss were recorded in 94 (100%) participants and for low grip strength and slowness, valid data were recorded for 91 (96.8%) participants. Weakness and slowness were the most common FP impairment criteria (84.6% and 57.1%, respectively), followed by poor energy and endurance (43.6%), low physical activity level (34%), and unintentional weight loss (16%). When evaluating the prevalence of frailty according to sex, it was observed that frail patients were mostly female (57.1% women vs. 37.3% men), unlike robust patients (2.9% women vs. 15.3% men).
Factors associated with frailty
Table 2, presents the characteristics and data of the frail and non-frail participants. Frailty was associated with age ≥ 80 years (p=0.004), and female sex (p=0.061). Frail patients presented a higher number of chronic diseases (p=0.002) and had higher atrial fibrillation (p=0.041), and cardiac insufficiency rates (p=0.071). We also found that frailty was associated with the aetiology of kidney disease, mainly with unknown and nonvascular aetiology. We could not find differences in educational level, social features, CIRS-G index or medication use, except in the use of erythropoietin, which was higher in frail patients, and the use of renin angiotensin system blockers (ARBs) which was higher in the nonfrail group. Regarding functional, cognitive and nutritional status, in bivariate analysis, frailty was associated with ADL disability (p<0.001), worse Barthel index scores (p<0.001), cognitive impairment (p=0.054) and risk or state of malnutrition (p=0.014). Physical performance tests showed poor performance in the SPPB test (p<0.001) and the slowest gait speed (p=0.061). Laboratory investigations showed that frailty was associated with hypoalbuminemia (p=0.034). However, we could not find differences in the degree of kidney dysfunction, anaemia or other nutritional markers.
| Sociodemographic characteristics | Non-frail Mean ± SD or n (%) |
Frail Mean ± SD or n (%) |
p |
|---|---|---|---|
| Age (years) | |||
| <80 | 33 (63.5) | 14 (33.3) | 0.004 |
| ≥ 80 | 19 (36.5) | 28 (66.7) | |
| Sex | |||
| Male | 37 (71.2) | 22 (52.4) | 0.061 |
| Female | 15 (28.8) | 20 (47.6) | |
| Educational level | |||
| <5 years | 21 (36.2) | 10 (23.8) | 0.186 |
| ≥ 5 years | 37 (63.8) | 32 (76.2) | |
| Social cohabitation | |||
| Live alone | 6 (10.3) | 8 (19.0) | 0.216 |
| Accompanied | 52 (89.7) | 34 (81.0) | |
| Comorbidity | |||
| Arterial hypertension | 48 (92.3) | 39 (92.9) | 0.92 |
| Diabetes mellitus type 2 | 21 (40.4) | 23 (54.8) | 0.165 |
| Sensory deficit | 19 (36.5) | 20 (47.6) | 0.278 |
| Neoplasm | 18 (34.6) | 14 (33.3) | 0.896 |
| Heart failure | 11 (21.2) | 16 (38.1) | 0.071 |
| Vascular peripheral disease | 11 (21.2) | 9 (21.4) | 0.974 |
| Ischemia heart disease | 9 (17.3) | 13 (31.0) | 0.12 |
| Atrial fibrillation or Atrial flutter | 8 (15.4) | 14 (33.3) | 0.041 |
| Stroke | 6 (11.5) | 7 (16.7) | 0.474 |
| COPD | 6 (11.5) | 9 (21.4) | 0.193 |
| CIRS-G Index | |||
| ≤ 2.5 points | 30 (57.7) | 21 (50.0) | 0.457 |
| >2.5 points | 22 (42.3) | 21 (50.0) | |
| Medication | |||
| Number of drugs | 8.65 ± 3.35 | 9.64 ± 3.32 | 0.157 |
| CKD aetiology | |||
| Vascular | 17 (32.7) | 5 (11.9) | 0.014 |
| Diabetic nephropathy | 12 (23.1) | 13 (31.0) | |
| Unknown | 10 (19.2) | 13 (31.0) | |
| Other causes | 7 (13.5) | 5 (11.9) | |
| Glomerular | 6 (11.5) | 2 (4.8) | |
| Chronic interstitial nephritis | 0 (0.0) | 4 (9.5) | |
| Physical function | |||
| Barthel Index | |||
| ≤ 90 | 10 (19.2) | 27 (64.3) | < 0.001 |
| >90 | 42 (80.8) | 15 (35.7) | |
| Disability for ADLs | |||
| No | 46 (79.3) | 19 (45.2) | <0.001 |
| Yes | 12 (20.7) | 23 (54.8) | |
| SPPB test | |||
| Normal | 42 (80.8) | 10 (25.6) | <0.001 |
| Poor performance | 10 (19.2) | 29 (74.4) | |
| Gait speed (m/s): | |||
| ≤ 0.8 | 43 (82.7) | 38 (97.4) | 0.06 |
| >0.8 | 9 (1.,3) | 1 (2.6) | |
| MoCA test: | |||
| <24 | 31 (59.6) | 29 (76.3) | 0.054 |
| ≥ 24 | 21 (40.4) | 9 (23.7) | |
| Nutritional status | |||
| MNA-SF | |||
| Normal | 37 (71.2) | 20 (48.8) | 0.014 |
| Risk or state of malnutrition | 15 (28.8) | 21 (51.2) | |
| BMI | 28.08 ± 4.18 | 27.52 ± 5.22 | 0.569 |
| Laboratory data | |||
| Hemoglobin | 11.9 ± 1.2 | 11.9 ± 1.4 | 0.8 |
| Creatinine | 3.3 ± 0.9 | 3.3 ± 0.9 | 0.828 |
| eGFR (CKD EPI) | 16.4 ± 4.4 | 16.1 ± 5.3 | 0.743 |
| Albumin | 3.9 ± 0.3 | 3.7 ± 0.5 | 0.034 |
| Prealbumin | 28.6 ± 12.3 | 29.1 ± 6.5 | 0.816 |
Table 2: Baseline clinical features in patients grouped by frailty status (n= 94).
Effect of frailty on clinical outcomes
At 1 year of follow-up, the mortality rate was 13%, and within the first 2 years, it was 24%. Figure 1, show a significantly increased risk of death in frail patients compared to prefrail and robust patients (p<0.001).
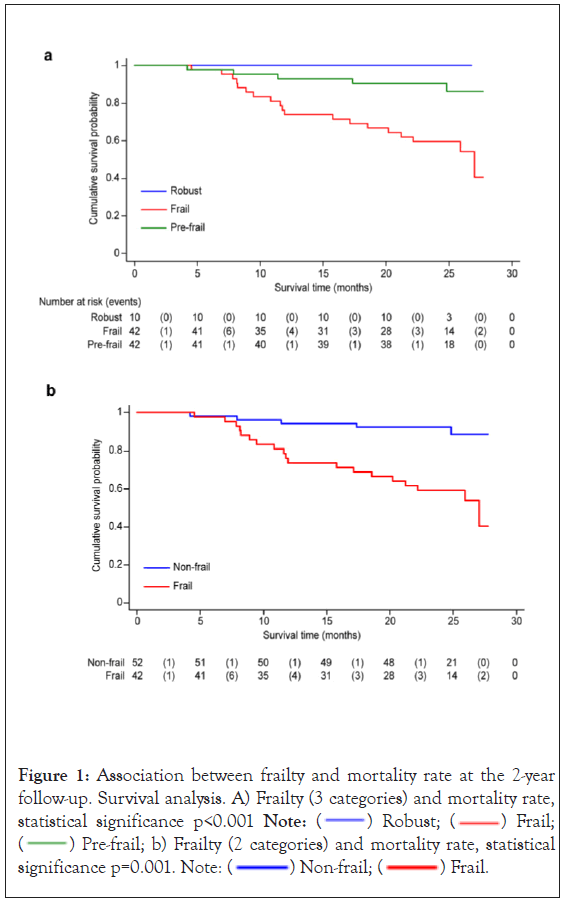
Figure 1: Association between frailty and mortality rate at the 2-year
follow-up. Survival analysis. A) Frailty (3 categories) and mortality rate, statistical significance p<0.001 Note:  Robust;
Robust;  Frail;
Frail;  Pre-frail; b) Frailty (2 categories) and mortality rate, statistical significance p=0.001. Note:
Pre-frail; b) Frailty (2 categories) and mortality rate, statistical significance p=0.001. Note:  Non-frail;
Non-frail;  Frail.
Frail.
To study the adjusted effect of frailty on the mortality rate, we included in the model those variables associated with frailty in bivariate analysis (p<0.10), that did not have collinearity. For the Cox proportional hazards model, functional tests were not included because they were considered components of frailty assessment (Barthel, SPPB and gait speed). The aetiology of kidney disease was also not included, due to the absence of data that relates the aetiology of CKD with the occurrence of frailty. Finally, the variables included in the predictive model were age, sex, cognitive impairment, nutritional status and frailty condition. Frailty was associated with an increased adjusted risk of death (HR 5.4; 95% CI: 1.859-15.866).
Among patients admitted to the hospital, during the first year of follow-up, 45% had at least one admission for medical reasons, and the median number of admissions was 2 (IQR 1-2). The most frequent primary conditions for which patients were admitted to the hospital were cardiovascular complications (57.8%), followed by infectious disease (46.7%), ictus (13.3%), bleeding (11.1%) and other causes (28.9%). Frailty was independently associated with a higher adjusted risk for hospital admission (OR 3.4; 95% CI: 1.247-9.534), mainly due to infectious disease (p=0.039) and bleeding (p=0.015). A trend towards admission due to cardiovascular complications (p=0.072) was also observed. Regarding the onset of RRT, within the first year of follow-up, 21 (22%) patients started dialysis, and during the 2-year follow-up period, 32 (34%) started dialysis. Of the latter, 28 patients (80%) underwent Haemodialysis (HD), and the rest underwent Peritoneal Dialysis (PD). The mean time to start dialysis was longer for robust patients (24.32 ± 1.99; 95% CI: 20.41-28.23) months than for frail patients (22.58 ± 1.34; 95% CI: 19.94-25.21) months, but these differences were not statistically significant (p=0.529). In multivariate analysis, after adjusting for age, sex, cognitive impairment and nutritional condition, there was no significant difference in the association between frailty and progression to dialysis therapy (HR 1.90; 95% CI: 0.64-5.64; p=0.249) (Figure 2A). However, when carrying out the analysis according to age, for patients over 80 ages a lower risk was observed for frail patients than for nonfrail patients (HR 0.09; 95% CI: 0.01-0.074; p=0.025) (p interaction 0.006). In contrast, for patients under 80 years, the risk of starting dialysis was higher for frail patients than for nonfrail patients (HR 2.2; 95% CI: 0.88-5.46; p=0.091) (Figures 2B and 2C). Concerning the performance of the frailty assessment tools, the Cox regression model showed a significant association between poor performance in the SPPB test and an increased risk of death at 2-years (HR 7.922; 95% CI: 2.686-23.369; p<0.001). In contrast, no association was observed between slow gait speed and the risk of death in these subjects (HR 1.435; 95% CI: 0.336-6.129; p=0.626). When assessing the ability of frailty tools to predict mortality using the c-statistic index score, the analysis showed a c-statistic index of 0.699 for FP (95% CI 0.602-0.795), 0.726 for the SPPB test (95% CI 0.636-0.817), and 0.527 for gait speed (95% CI 0.477-0.576). The comparison of the predictive ability of the FP and SPPB tests did not show significant differences (p=0.510).
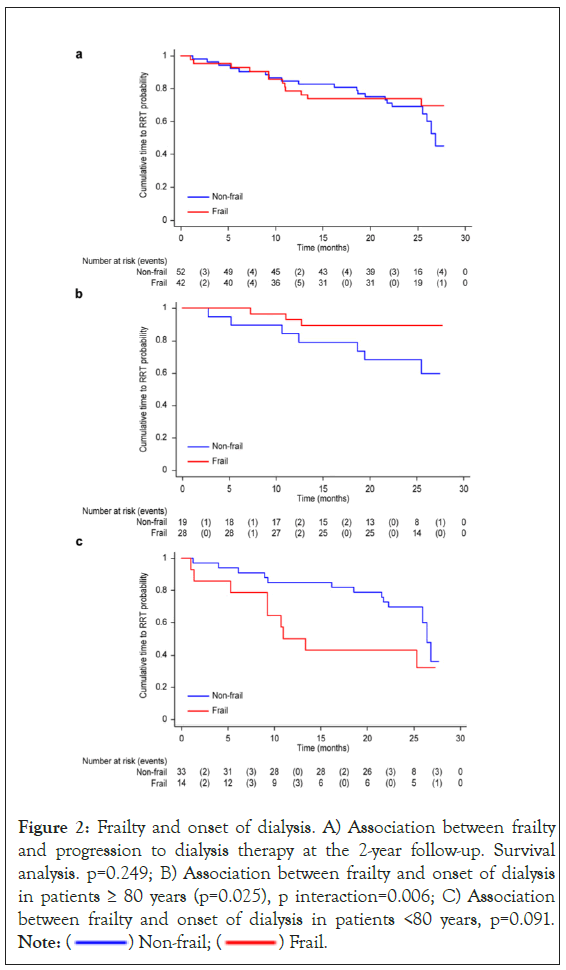
Figure 2: Frailty and onset of dialysis. A) Association between frailty
and progression to dialysis therapy at the 2-year follow-up. Survival
analysis. p=0.249; B) Association between frailty and onset of dialysis
in patients ≥ 80 years (p=0.025), p interaction=0.006; C) Association
between frailty and onset of dialysis in patients <80 years, p=0.091. Note:  Non-frail;
Non-frail;  Frail.
Frail.
This prospective study of older patients with advanced non-dialysis CKD aims to determine the effect of frailty in this especially vulnerable population on the occurrence of serious adverse outcomes other than mortality, such as progression to dialysis and risk of hospitalization. In our study, we also describe the prevalence of frailty, identify characteristics associated with frailty and assess agreement between different frailty assessment tools used in clinical practice. The prevalence of frailty, based on the Fried criteria, is high in the older adult population with CKD. Even though the prevalence of this condition in CKD patients varies depending on the stage of disease and the assessment tool used [1,13-15], the prevalence of frailty in our cohort (44.5%) was in keeping with that reported in similar populations. Previous studies in community-dwelling elderly patients found a lower prevalence, in the range of 7-25% [16-19]. The prevalence is higher among patients with kidney disease, with a range of 7-51.2% [1,3,14,20], likely due to the high prevalence of cardiovascular risk factors and vascular disease, increased risk of malnutrition, bone mineral disease, accentuated phenomena of inflammation, and other factors not yet identified, which together favors the development of this condition [2-5,13,20,21].
As in previous studies, patients with CKD were mostly male and presented high comorbidity, especially cardiovascular disease, arterial hypertension and diabetes mellitus [1,16,22]. Further analysis explored the association between frailty phenotype, high comorbidity and physical disability, showing the presence of at least one of them in 69% of patients and three of them in 14%. Comorbidity was the most frequent condition observed, followed by frailty and physical disability. We emphasize the importance of distinguishing each one. Although they often overlap in clinical practice, they should not be used interchangeably. Figure 3, displays the overlap between these characteristics.
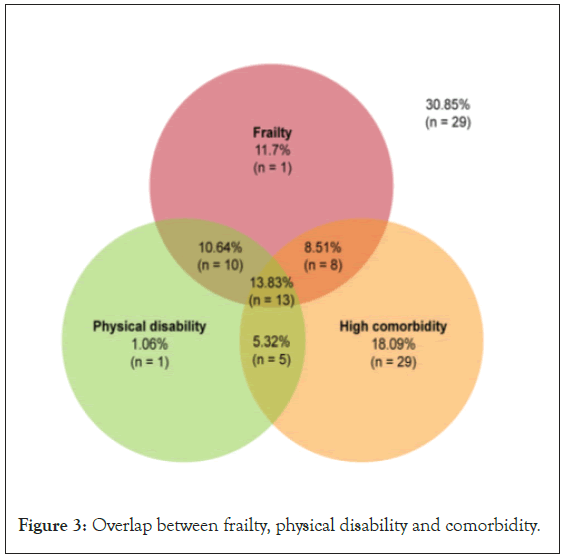
Figure 3: Overlap between frailty, physical disability and comorbidity.
The analysis of frailty criteria shows that weakness (85%) and slowness (57%) were the most prevalent criteria in our sample. Both criteria were also the most prevalent in the study by Fried et al., but with a lower prevalence of approximately 20% each, along with low physical activity (22%). In the CKD population, even though there are only a few studies that have analyzed frailty conditions using the original Fried’s criteria, the study by Wilhelm-Leen et al. showed that both criteria, weakness and slowness, were also the most prevalent. These results highlight the involvement of muscle structure, as part of the mechanisms of chronic inflammation, malnutrition-inflammation and impaired metabolic functions that occur frequently in CKD and can explain the high prevalence of sarcopenia in these subjects [1,23,24].
We found that frailty was more prevalent among individuals over 85 years of age than among those between 65 and 85 years of age, and females, as shown in other studies [25-27]. There was no significant difference in comorbidity measured by the CIRS-G index, or in the number of drugs being used. The greater use of erythropoietin in frail patients, was probably related to the higher prevalence of symptomatic anaemia in these subjects. Our findings are similar to the results from previous studies suggesting that frailty has a large impact on health because of its strong association with malnutrition and cognitive impairment in older patients with kidney disease. This latter association has been found in so many international epidemiological studies [3,5,16,28], that cognitive impairment has been proposed as part of the FP. This could be explained by the strong association between frailty and vascular disease, as well as the higher risk for Alzheimer’s disease in these patients [3,29,30].
Malnutrition also has a significant negative impact on the evolution of patients with kidney disease and is considered an important predictor of morbidity and mortality [28,31,32]. Among the mechanisms studied, protein restrictions in the diet and Protein-Energy Wasting (PEW) syndrome are linked not only to worse nutritional status in the elderly, but also to the occurrence of adverse events. Hypoalbuminemia has been used as a representative factor for PEW syndrome in patients with CKD. Among the nutritional tests, the short version of the MNA has been considered a useful method and validated for the assessment of malnutrition in the elderly in different clinical scenarios [33].
Regarding frailty and clinical outcomes in the CKD population, there is less evidence than in the general population. Studies published to date have shown an association between frailty and increased mortality, with results that vary depending on the stage of CKD [1,13,34-36]. Most of these studies have been based on a modification of Fried’s criteria and carried out in younger subjects, with less functional impairment than in our study, which makes it difficult to make comparisons. An interesting finding of our study is that it shows how age affects the relationship between frailty and the onset of dialysis. The analysis in the youngest group (<80 years) shows that frailty favors the onset of dialysis in this group of patients. This finding corroborates that reported by Roshanravan et al. in the middle-aged population, in whom frailty was associated with the combined outcome of death or start of dialysis, with a higher risk in those with an eGFR of<30 ml/min. The opposite effect has also been described in the oldest group (≥ 80 years), especially those with high comorbidity and greater functional impairment, for whom conservative management has been proposed [37-39].
Hospital admission has been considered a risk factor for loss of functionality in elderly individuals and is related to the development of geriatric syndromes (delirium, falls, incontinence, immobility syndrome, constipation, ulcer pressure and polypharmacy), nosocomial infections, depression, drug‒drug interactions and adverse drug events [16,35,36,40]. All of these factors may lead to prolonged hospitalization, increased morbidity and mortality, and health costs. In our study, we found a strong association between frailty and hospital admission, but also with the highest number of hospital admissions in frail patients with kidney disease (p=0.002).
Even when comprehensive geriatric assessment has become the internationally established method to assess older people in clinical practice, the practical limitation of the assessment is the time and expertise needed [41], hence the importance of finding equally reliable but more efficient methods for routine care. To date, there are many tools to screen for and diagnose or assess frailty in an individual person. Screening for frailty seems to be more feasible when focusing on physical frailty, as a first step in clinical management [14,30,42-45]. However, since older patients with advanced CKD usually have a lower level of physical activity, which can increase the detection of frailty in these subjects, it is very important to choose reliable functional measures.
We compared the usefulness of two assessments, SPPB scores and gait speed, both of which have been widely used to identify patients at higher risk of adverse events, with similar efficacy in patients with chronic diseases, but whose diagnostic accuracy in the advanced CKD population has not been well established. We found that the SPPB test had better predictive ability in estimating the risk of death at two years, without significant differences with respect to that obtained by the Fried criteria.
The distinction of frail people with advanced CKD from those who are not frail should therefore be an essential part of assessment in these older patients who might result in the implementation of early interventions and follow-up. Therapeutic strategies based on the prescription of low- intensity resistance and aerobic exercise, vitamin D supplementation, adequate caloric and protein intakes, cognitive training activities and avoidance of polypharmacy may prevent or delay the onset of frailty and improve clinical outcomes.
We thank Dr. Carlos Verdejo Bravo for providing invaluable guidance and support in the completion and success of this study. We also thank Manuel Enrique Fuentes Ferrer for the statistical analysis, suggestions and cooperation.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Portilla-Franco ME, Tornero-Molina F, Herrero-Calvo JA, Gil-Gregorio P (2024) Frailty among Older Patients with Baseline Advanced Non- Dialysis Chronic Kidney Disease. J Clin Trials. 14:558.
Received: 27-Feb-2024, Manuscript No. JCTR-24-29798; Editor assigned: 01-Mar-2024, Pre QC No. JCTR-24-29798(PQ); Reviewed: 15-Mar-2024, QC No. JCTR-24-29798; Revised: 22-Mar-2024, Manuscript No. JCTR-24-29798(R); Published: 29-Mar-2024 , DOI: 10.35248/2167-0870.24.14.558.
Copyright: © 2024 Franco MEP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.