
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2015) Volume 4, Issue 1
The biosorption mechanism of metal removal (copper, Cu and zinc, Zn) by four phytoremediation macrophytes biomasses including sunflower (Helianthus annuus), Chinese cabbage (Brassica campestris), cattail (Typha latifolia), and reed (Phragmites communis) was investigated in this study. The primary objectives were exploring the potential of reusing these bio-wastes after harvesting from phytoremediation operations. Based on the surface area, zeta potential, scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) investigations, Chinese cabbage biomass presented the highest metal adsorption property while both cattail and reed revealed a lower adsorption capability for both metals tested. The equilibrium adsorption rate between biomass and metal occurred very fast during the first 10min. The metal adsorption data were fitted with the Langmuir and Freundlich isotherms and presented that the Langmuir isotherm was the best fitted model for all biomass tested. All tested biomasses are fast growing plants with fairly high biomass production that are able to accumulate metals. The Langmuir model was used to calculate maximum adsorption capacity and related adsorption parameters in this study. The results revealed that the maximum metal adsorption capacity Qmax was in the order of Chinese cabbage (Cu: 2000; Zn: 1111 mg/kg) > sunflower (Cu: 1482; Zn: 769 mg/kg) > reed (Cu: 238; Zn: 161 mg/kg) > cattail (Cu: 200; Zn: 133mg/kg). The harvested sunflower, Chinese cabbage, cattail, and reed biomass possess the potential to be employed as biosorbents to remove Cu and Zn from aqueous solutions. Adsorption isotherms derived in this study might be crucial information for practical design and operation of adsorption engineering processes and prediction of relation between reused macrophyte biosorbents and heavy metal adsorbates.
<Keywords: Heavy metals; Biosorbent; Macrophyte; Adsorption; Phytoremediation
The wastewater generated from confined swine operations is one of the primary pollution sources in Taiwan [1,2]. The effluent is discharged in the surrounding waterways containing significant amounts of heavy metals such as copper (Cu) and zinc (Zn). These metals are intentionally added in fodder to prevent diarrhea and to enhance immune systems of swine. Conventional physical-chemical technologies employed for heavy metals removal for contaminated water include chemical precipitation, ion-exchange, however, they are usually quite costly and energy consumed. Phytoremediation using green plants in constructed wetlands and soil decontamination recently has drawn great attention in Taiwan and worldwide [3-5].
The biomass can be harvested and used for various purposes such as biosorbents for metal removal in water treatment [6,7]. The use and evaluation of recycled biosorbents is very important to compare and analyze the adsorption mechanism and optimize the purification techniques that are based on biosorption. Several studies were published recently using recycled bio-wastes to remove pollutants [8-10]. The use of recycled and dried plants for metal removal as a simple biosorbent material has advantages in its efficiency in detoxifying dilute effluents and has been viewed as a cost-effective and energy-efficient wastewater treatment approach. The reuse of harvested macrophytes in wastewater engineering can also benefit waste disposal management and save waste treatment costs. The adsorption properties of phytoremediation macrophytes have been investigated for the removal of metals in polluted effluent. The results revealed that the extent of metal adsorption onto biomass seems to have important consequences in the capacity of metal removal [11]. Therefore, it is important to investigate the biosorption mechanism and related sorption parameters of harvested macrophytes to facilitate future biosorbent water purification operation. Metal cations in polluted effluent can be adsorbed by the negative charge of the macrophyte biomass surface. The process of metal removal by plants involves a combination of rapid sorption on the cell wall surface and slow accumulation and possibly translocation into the biomass [12]. The rapid sorption may include chelation and ion exchange. Carboxylic group, one of the functional groups on the plant biomass surface, provides binding sites with metals [13].
Research results indicated that all plant parts might accumulate heavy metals, and the ability to concentrate metals from the external solution varied between both plant parts and metals. Be twe e n 24% and 59% of the metal content was adsorbed onto the cell walls of the plants [14]. The biomasses of plants, both living and dead, were heavy metal accumulators. The mechanisms of metal biosorption included extracellular accumulation, cell surface sorption, and intracellular accumulation. These mechanisms resulted from complexation, ion exchange, precipitation, and adsorption [15]. The main mechanism involved in biosorption was reported as ion exchange between metal cations and counterions presented in the macrophytes biomass. The investigation revealed that no significant difference was observed in the exchange amounts while using muti-metal or individual metal solutions [16]. Sunflower (Helianthus annuus) and Chinese cabbage (Brassica campestris) are fast-growing crops that have been commonly used for phytoextraction of metal contaminated soils, while reed (Phragmites communis) and cattail (Typha latifolia) are predominant macrophytes that have been employed for water purification within constructed wetlands. These plants contain high amount of lignin and cellulose which may adsorb heavy metal cations from aqueous solution. After harvesting, these plant biomasses could be used as biosorbents for metal adsorption. Brassica family has been reported for its prominent ability to remove heavy metals from contaminated soils [17]. B. campestris and H. annuus have the potential as biofuel to become the substitute of fossil fuels, especially the increasing oil prize in recent years. The higher biomass production of these economic crops, namely sunflower and Chinese cabbage, contribute them being the candidates of phytoextration contaminant and then harvested as potential biosorbents. Reed and cattail, commonly used macrophytes in constructed wetlands for water pollution mitigation, have been reported as a very high adsorption affinity value, which assist to predict its high ability to adsorb heavy metals in aqueous solutions [18]. This study focused on the biosorption characteristics of the harvested biomass of plants may provide information for enhancing phytoremediation processes to remove metals both in soil and water. The aim of this study was to investigate the biosorption performance and mechanisms of four macrophyte biomasses.
The benefits from this study were two folds: to highlight the metal adsorption capability of plant biomass for environmental decontamination, and to test the possibility to recycle the harvested biomass for biosorbents.
The preparation of harvested macrophytes for metal adsorption experiments
The plant biomasses collected from local soil contaminated sites and constructed wetlands were rinsed with deionized water. Fresh biomass was dried in an oven at 104ºC for 24 h and grinded. T h e grinded biomass was passed through 200 and 250 mesh (74 - 62 μm) of filters for the surface area determination, zeta potential measurement, and sorption experiments. Analytical grade chemicals were used to prepare metal stock solutions. The natural pH of the synthetic solutions was measured to be 5.3. This pH was maintained throughout for all the experiments except for zeta potential measurement. The zeta potential were conducted at pH 2 to 10 varied with NaOH and HCl. Triplicates of 0.2 g grinded biomass and 25 mL
metal solution of intended concentration were performed in 50 mL flask. Adsorption tests were conducted at room temperature (24±2ºC). Flasks were shaken on a rotary shaker under various contact time (e.g. 10, 30, 60, 120, 180, and 360 min) to evaluate the metal adsorption capacity. The solution was separated by centrifugation with 4000 rpm for 20 min. The supernates were then collected and analyzed for metal contents by an atomic adsorption spectrophotometer.
The feasible adsorption isotherms fitting
Adsorption experiments were conducted to determine the adsorption of Cu and Zn by the studied plants. The adsorption capacity Q, the amounts of total metals adsorbed per biomass unit was evaluated using the following formula:
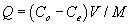
Where Co is the initial metal concentration (mg/L), Ce is the equilibrium concentration (mg/L), V is the volume (L) of metal solution, and M is the biomass (g) of the plant tested. The adsorption of metals by biosorbents was further evaluated using the Freundlich and Langmuir adsorption isotherms. The Freundlich equation can be written as
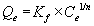
In the aforementioned equation, Qe is the metal content onto the adsorbent material, mg/kg; Kf is an empirical constant related to the adsorption capacity; Ce is the equilibrium metal concentration in solution; and n is a constant related to the intensity of adsorption. The Langmuir equation can be written as
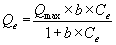
In the equation, Qmax is the maximum metal capacity, mg/kg; and b is a parameter related to the binding strength of metals.
Scanning electron microscopy (SEM)-energy dispersive X-ray (EDX) spectroscopy
Pretreated macrophyte samples were gold-coated for SEM observation with qualitative EDX analysis. Specifically, grinded and dried samples were mounted on carbon tape and sputter coated in gold. A Hitachi S-4300 SEM (Tokyo, Japan) was used to capture micrographs. The elements C, O, Cu, and Zn were detected using a SEM coupled with an EDX spectroscopy at an acceleration voltage of 15 kV.
The properties of tested macrophytes
The surface areas of four studied biomasses were 2.75 ± 0.48, 3.71 ± 0.13, 2.30 ± 0.03, and 2.43 ± 0.17 m2/g, for sunflower, Chinese cabbage, cattail, and reed, respectively, analyzed by the BET method with liquid N2. Chinese cabbage, the Brassica family, has the largest surface area in this study rendering for better metal adsorption. The adsorption capacity can be further illustrated via comparing the electrokinetic potential (zeta potential) as shown in Figure 1. The effect of pH on the zeta potential of all tested macrophytes was examined. The zeta potential had negative charge for all studied macrophytes rendering for the potential of metal adsorption. The increase in negative charge of the zeta potential was observed while the pH increased. This result indicated that the degree of metal biosorption may increase as the pH increased. The biomass Chinese cabbage was recorded as the negative zeta potential around neutral pH while the lowest recorded was at pH 10. This result revealed that Chinese cabbage had better metal adsorption capability compared to other macrophytes tested. The rest of tested plants also presented negative charge of zeta potential following the order sunflower < reed < cattail. The lower negative zeta potential also indicated better metal cations adsorption.
The metal adsorption rate and isotherms
The adsorption rate of Cu and Zn by four studied biomasses is depicted in Figure 2. Most of metal biosorption occurred during first 10 min. This adsorption result revealed that a contact time of 120 min for both Cu and Zn was sufficient to achieve equilibrium for four tested macrophytes. Similar rapid metal biosorption has been reported by other researcher [19]. Several factors including the structure of biosorbent and existence of metal species have also been presented to influence adsorption rates. In order to obtain basic information of tested macrophytes as biosorbents, the equilibrium metal concentration (Ce) and the concentration adsorbed onto the surface of the biomass (Q) were linearized and fitted to the Langmuir and Freundlich equations. The Langmuir and Freundlich isotherm models were calculated to determine the adsorption capacities and related parameters. The calculation results and related Langmuir and Freundlich sorption parameters are listed in Table 1. The sorption process for Cu and Zn by four tested biomasses was better described by the Langmuir equation (R2 = 0.90-0.99) compared to the Freundlich model (R2 = 0.67-0.97). The linear regression was calculated to demonstrate that the Langmuir equation was best fitted, therefore, the sorption as a monolayer can be assumed. The maximum sorption capacity Qmax of Cu was 1482, 2000, 200, and 238 mg/kg while the Qmax of Zn was 769, 1111, 133, and 161 mg/kg for biomass sunflower, Chinese cabbage, cattail, and reed, respectively, predicted by the Langmuir model. The aforementioned maximum sorption capacity was comparable with that of the activated carbon and less than that of the tested biosorbent peanut hulls [20]. The related adsorption parameters were also calculated through the Langmuir equation. For Cu, the binding constant b was 3.00, 3.80, 0.42, and 0.46 for biomass sunflower, Chinese cabbage, cattail, and reed, respectively. For Zn, the binding parameter b was 2.92, 5.11, 0.51, and 0.54 for biomass sunflower, Chinese cabbage, cattail, and reed, respectively. The high b value of Chinese cabbage biomass is reflected by the steep initial slope of the adsorption isotherm which indicated a high affinity for the adsorbate in dilute metal solutions. Research has presented that wetland macrophyte, Ceratophyllum demersum, was an effective biosorbent for Zn and Cu removal under dilute metal conditions. Batch adsorption experiments showed that the Langmuir isotherm was best fit model and the maximum adsorption capacity was 13.98 mg/g for Zn and 6.17 free floating macrophyte Lemna minor biomass regarding its adsorption of metals from aqueous solutions. The equilibrium adsorption was reached within 40-60 min. The maximum adsorption capacities of biomass was determined as 83 mg/g for Cu based on the best fitted Langmuir equation [21]. The maximum adsorption capacity might vary with the biomass investigated and adsorption experimental conditions. The equilibrium metal concentration (Ce) after a contact time of 5 h was lower than the initial concentration (Ci). Five hours was assumed to be adequate for the adsorption system to achieve equilibrium which was longer than the time (60 min) to reach equilibrated condition in the aforementioned adsorption rate experiment. The removal efficiency of metals from solutions can be expressed as the fraction of metals adsorbed by studied biomasses which was related to the reciprocal value of the ratio of the metal concentration in the solution at equilibrium to that in the initial solution. In general, the fraction of metals adsorbed onto biomass decreased as the initial concentration Ci increased. At high initial Cu concentration (10 mg/L), the percentage of Cu that was adsorbed by the biomass decreased to around 20% then gradually leveled off for both cattail and reed while sunflower and Chinese cabbage continued to drop. At high initial Zn concentration (5 mg/L), the percentage of Zn that was adsorbed by the biomass decreased to around 18% then gradually leveled off for both cattail and reed while sunflower and Chinese cabbage gradually decrease. At low initial Cu concentration (1 mg/L), the metals adsorbed by the biomass ranged 72% for sunflower, 73% for Chinese cabbage, 61% for cattail, and 67% for reed, respectively, while at low initial Zn concentration (1 mg/L), the metals adsorbed by the biomass ranged 50% for sunflower, 54% for Chinese cabbage, 35% for cattail, and 40% for reed, respectively. The biosorption efficiency was high at a low metal concentration, especially for Chinese cabbage and sunflower. At a low metal concentration, the ratio of available adsorbent surface area to the metal in solution was high indicating a great metal removal. As metal initial concentration increased, the efficiency was gradually decreased. This result might be attributed to the saturation of the adsorption sites on the biomass.
| (a) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Copper | Qmax | b | R2 | Kf | N | R2 |
| Sunflower | 1482.57 | 3.00 | 0.99 | 297.85 | 1.33 | 0.95 |
| Chinese cabbage | 2000.00 | 3.80 | 0.99 | 350.91 | 1.29 | 0.96 |
| Cattail | 200.00 | 0.42 | 0.92 | 92.96 | 4.29 | 0.72 |
| Reed | 238.10 | 0.46 | 0.90 | 103.23 | 4.20 | 0.70 |
| (b) Zinc | ||||||
| Sunflower | 769.23 | 2.92 | 0.99 | 145.21 | 1.58 | 0.96 |
| Chinese cabbage | 1111.11 | 5.11 | 0.99 | 143.91 | 1.38 | 0.97 |
| Cattail | 133.33 | 0.51 | 0.98 | 66.65 | 4.18 | 0.80 |
| Reed | 161.29 | 0.54 | 0.94 | 82.57 | 4.41 | 0.67 |
Table 1: The adsorption parameters of linearized Langmuir and Freundlich isotherms for four macrophyte biomasses.
The microstructure investigation
The microstructures of the tested biosorbents and adsorbed metal determinations onto biomass surface were performed by the scanning electron microscopy (SEM) and energy dispersive X-ray (EDX). The biomass treated with metals revealed several small bulges that were not observed before the metal sorption experiment. Further EDX observations indicated that small bulges are higher in Cu and Zn. There were more bulges on the surface of Chinese cabbage compared to other three studied macrophytes. The results also suggested that Chinese cabbage might have better metal sorption capacity.
The harvested biomass of sunflower, Chinese cabbage, cattail, and reed possesses the potential to be used as biosorbents to remove metals from aqueous solutions. Adsorption experiment results showed that Cu and Zn adsorptions were fairly rapid occurring within first 10min. The adsorption capability of four tested biomasses can be well predicted by the Langmuir adsorption model. The surface area, zeta potential, SEM, and EDX results revealed that Chinese cabbage biomass presented the highest metal adsorption property while both cattail and reed presented lower adsorption capability for both metals tested. Further study (e.g. FT-IR) might be required to scrutinize the chemical functionalities responsible for the adsorption of the heavy metals. These studied plant biomasses are natural abundant and can be recycled from environmental decontamination operations, namely phytoremediation of metal polluted soil and water purification within constructed wetlands. This research results can benefit adsorption process engineering for mitigation of polluted metal water by reusing harvested macrophytes.