Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 4
Background: Increased standardization may be necessary to maximize benefits and minimize complications with fullface
rejuvenation using hyaluronic acid (HA) fillers. The aim of this study was to assess the utility and safety of the
standardized MD Codes approach.
Methods: This was a retrospective review of data from 84 patients receiving full-face rejuvenation with HA fillers
using the MD Codes technique within a single treatment plan, across two visits separated by ~1 month.
Results: Participants had a mean age of 49.1 ± 9.9 years, and 83 (98.8%) were female. They were treated with 527
syringes of filler (6.3 ± 1.8 per patient): 334 syringes (63.4%; 4.0 ± 1.5 per patient) during visit 1 and 193 (36.6%; 2.3
± 1.1 per patient) during visit 2. The most commonly treated areas were the cheek (n=84; 100%) and nasolabial fold
(n=26; 31.0%) at visit 1, and the nasolabial fold (n=36; 43.9%) and tear trough (n=35; 42.7%) at visit 2. Eight adverse
events were recorded in 6 patients (7.1%): bruising, n=4; pain, n=4. All were minor and transient. There were no lateonset
complications.
Conclusion: Full-face rejuvenation using the MD Codes technique can be successfully performed with minimal
complications. This approach may define a new standard of care.
Facial filler; Full-face rejuvenation; Hyaluronic acid; MD codes; Vycross
Aging of the face is a multifaceted process. A key factor is that facial volume naturally declines over time as fat and underlying bone diminish [1,2]. However, there are many other influences, including the effects of gravity and facial muscle movement, as well as extrinsic factors such as smoking and exposure to ultraviolet radiation [1,2].
The emergence of stable fillers with superior lift capabilities has opened the door to replacing lost volume, restoring proportions, and reducing the impact of wrinkles. It has also been suggested that fillers can be used to modulate muscle movement in the face [3].
The ideal facial filler product would be one that is easy to use, leads to minimal complications, and gives natural results that are long-lasting but not permanent.
The VycrossTM range of hyaluronic acid (HA) fillers provide a versatile portfolio of products designed to address the needs of all areas of the face. These fillers, based on 10% high-molecularweight and 90% low-molecular-weight HA, contain varying concentrations to achieve different levels of firmness (elastic modulus; G’) [4].
This optimizes the gel properties of each product for different areas and planes of the face. The safety, efficacy and durability of results with these treatments have been demonstrated in a number of studies [5-13].
Recent years have seen a progression towards a more ‘full-face approach ’ to treatment. Inevitably, this often necessitates multiple syringes of filler being used during a single treatment session, and raises questions about which products to use in which areas of the face, the optimal technical specifications (injection depths, volumes, techniques, etc.), and the associated risk of the complications. Greater standardization across practitioners may be required to maximize the benefits of this approach.
In an attempt to set such standards for the process of facial rejuvenation with injectables, the ‘Aesthetic Leaders in Facial Aesthetics Consensus Committee ’ conceived a series of publications providing practical recommendations on facial assessment and injection technique [14-16]. Furthermore, the lead author of those publications, Dr Mauricio de Maio, has developed and taught the use of MD CodesTM.
These specify precise treatment subunits within each facial area, and provide experience-based guidance on the favored HA filler G’ and volume range for each subunit, injection device (needle or cannula), injection delivery method (micro-aliquot, aliquot, small bolus, linear and fanning), target structures for delivery of the product (dermal, mucosal, subcutaneous or supraperiosteal), and key safety issues. Importantly, while the MD Codes postulate a standardized framework, they also give practitioners considerable scope to exercise clinical judgment when customizing the treatment plan to individual patient needs.
Here, we report our experience of full-face rejuvenation with HA fillers, based on a single treatment plan using the MD Codes technique.
Study design and participants
This was a retrospective review of data from patients receiving full-face rejuvenation with HA-based fillers, treated according to the MD Codes technique at a single center between June 2017 and December 2018. The study was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Eligible patients were adult males and females wishing to receive non-surgical facial correction using fillers. Individuals were excluded if they had undergone previous treatment within the past 3 months or had an allergy to any of the study products, wound healing problems, excessive sun damage, or psychiatric conditions/comorbidities that could interfere with treatment. Women who were pregnant or breastfeeding were also excluded.
Procedures
All patients were treated using the MD Codes approach in a single treatment plan (Figure 1), with injections given across two visits. In most cases, visit 2 was around 1 month after visit 1, but this varied between 3 days and 3 months according to the details of the treatment plan and individual patient availability.
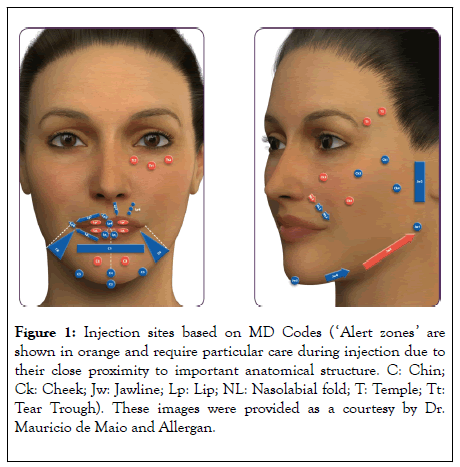
Figure 1: Injection sites based on MD Codes (‘Alert zones’ are shown in orange and require particular care during injection due to their close proximity to important anatomical structure. C: Chin; Ck: Cheek; Jw: Jawline; Lp: Lip; NL: Nasolabial fold; T: Temple; Tt: Tear Trough). These images were provided as a courtesy by Dr. Mauricio de Maio and Allergan.
The fillers used were from a single range of HA-based products (Allergan, Dublin, Ireland): VYC-20 (Voluma®; 20 mg/mL HA), VYC-17.5 (Volift®; 17.5 mg/mL HA), VYC-15 (Volbella®; 15 mg/mL HA), and VYC-12 (Volite®; 12 mg/mL HA). When indicated, botulinum toxin type A (BoNTA; onabotulinumtoxinA, Vistabex®, Allergan) was also used in the upper third of the face (glabellar, crow’s feet and forehead lines).
The facial areas treated with filler were the temple, tear trough, cheek, nasolabial fold, lip, chin and jawline (Figure 1). Specific treatment areas and injection points were determined individually for each patient based on their stated requirements and budget and the clinical judgment of the physician. Broadly, the temple was treated with VYC-20 (T1: 0.7 mL; T2: 0.3 mL), given supraperiosteally, using a needle and bolus technique, and the tear trough was treated with VYC-15 (Tt1-2-3: 0.3-0.5 mL) using a cannula and a micro-aliquot technique.
The cheek was treated with VYC-20 and/or VYC-17.5 (Ck1: 0.3-0.4 mL; Ck2: 0.2–0.3 mL; Ck3: 0.3-0.4 mL; Ck4: 0.5 mL) using a needle or cannula with a small bolus or fanning technique; injections were supraperiosteal or subcutaneous. The nasolabial fold was injected in the dermis in a linear manner, using VYC-17.5 (NL1-2-3: 0.3-0.5 mL) and a needle or cannula. The lip was treated with VYC-17.5 and/or VYC-15 (Lp1-8: 1.0 mL) using a needle or cannula, with the product injected into the dermis or mucosa.
Finally, the chin and jawline were each injected with VYC-20 or VYC-17.5 (C1: 0.3-0.5 mL; C2: 0.2-0.5 mL; C4: 0.2-0.4 mL; C5: 0.4 mL; C6: 0.3-0.5 mL; Jw1: 0.2-0.4 mL; Jw3: 0.5 mL; Jw4: 0.2 mL) in a subcutaneous and/or supraperiosteal manner, using a cannula. No topical anesthesia was used before filler treatment because the products contain lidocaine.
Where necessary, VYC-12 was used for the correction of fine lines or for skin quality improvements. It was always injected separately, usually between treatment sessions based on the MD Codes approach, with the aim of enhancing the overall aesthetic improvement of the face.
Assessments
Baseline characteristics (age and sex) were recorded for all patients. Data collected included the facial areas treated and the number of syringes of filler used during each treatment session for each patient.
Adverse events (AEs) were assessed during both treatment and follow-up visits; between visits, participants were also encouraged to provide details of any AEs over the telephone. Patients were followed for up to 18 months.
Statistical analysis
Descriptive statistics are provided as appropriate, including mean, standard deviation and range for continuous variables, and frequency and percentage for categorical variables.
Treatments
A total of 84 patients were included in the study: 83 females (98.8%) and 1 male (1.2%). The mean age was 49.1 ± 9.9 years (range: 25-68 years) (Table 1). Patients were treated with a total of 527 syringes of filler. This equates to a mean of 6.3 ± 1.8 syringes per patient (range: 2-13). The majority of syringes were used during treatment visit 1 (334/527; 63.4% of the total), with a mean of 4.0 ± 1.5 syringes per patient (range: 2-8). At the data cut-off, 82 patients had already completed treatment visit 2. In this session, patients were injected with a total of 193 syringes (36.6% of the total), with a mean of 2.3 ± 1.1 syringes per patient (range: 1-5).
| Variable | Patients, N=84 |
|---|---|
| Age, years, mean ± SD (range) | 49.1 ± 9.9 (25–68) |
| Sex, n (%) | |
| Female | 83 (98.8) |
| Male | 1 (1.2) |
| Filler usage, mean syringes per patient ± SD (range) | |
| Total | 6.3 ± 1.8 (2–13) |
| Visit 1 | 4.0 ± 1.5 (2–8) |
| Visit 2 | 2.3 ± 1.1 (1–5) |
SD: Standard Deviation.
Table 1: Patient characteristics and treatment overview.
At treatment visit 1, all 84 patients were injected in the cheek (Figure 2). Other commonly injected areas included the nasolabial fold (n=26; 31.0%), chin (n=15; 17.9%), jawline (n=10; 11.9%), and tear trough (n=10; 11.9%). At treatment visit 2, there was a greater spread of treatment areas, with the most common being the nasolabial fold (n=36; 43.9%), tear trough (n=35; 42.7%), chin (n=27; 32.9%), lip (n=17; 20.7%), and jawline (n=15; 18.3%).
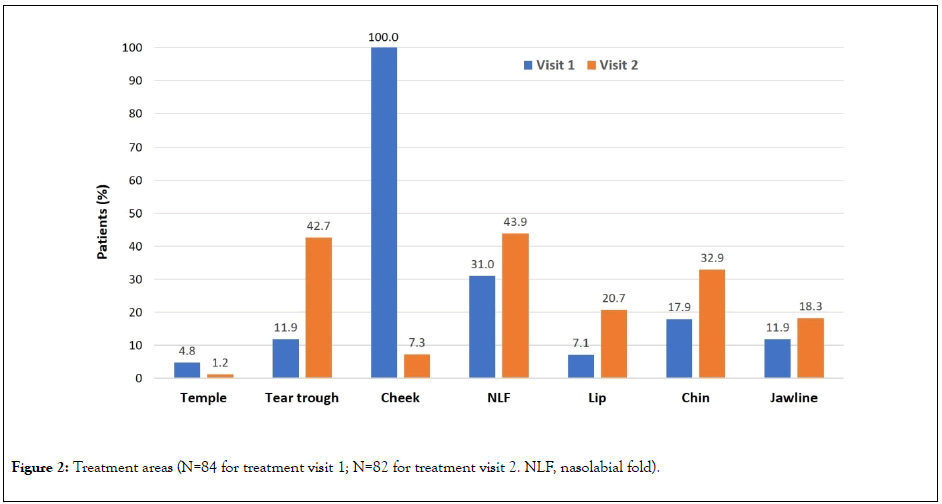
Figure 2: Treatment areas (N=84 for treatment visit 1; N=82 for treatment visit 2. NLF, nasolabial fold).
Twenty-four patients (28.6%) were also treated with VYC-12 to correct fine lines or improve skin quality, and 54 (64.3%) were treated with onabotulinumtoxin A in the upper third of the face (glabellar, crow’s feet and forehead lines).
Sample before-and-after images of patients receiving full-face treatment based on the MD Codes approach are shown in Figures 3 and 4.
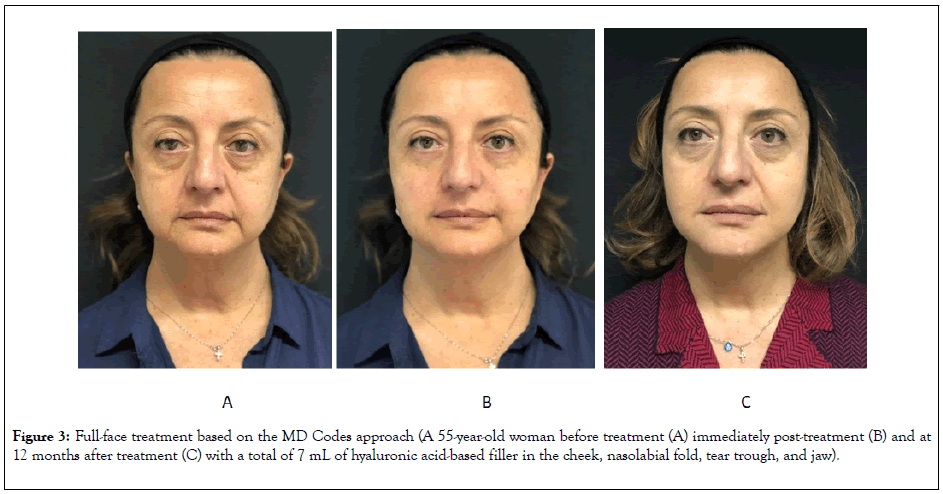
Figure 3: Full-face treatment based on the MD Codes approach (A 55-year-old woman before treatment (A) immediately post-treatment (B) and at 12 months after treatment (C) with a total of 7 mL of hyaluronic acid-based filler in the cheek, nasolabial fold, tear trough, and jaw).
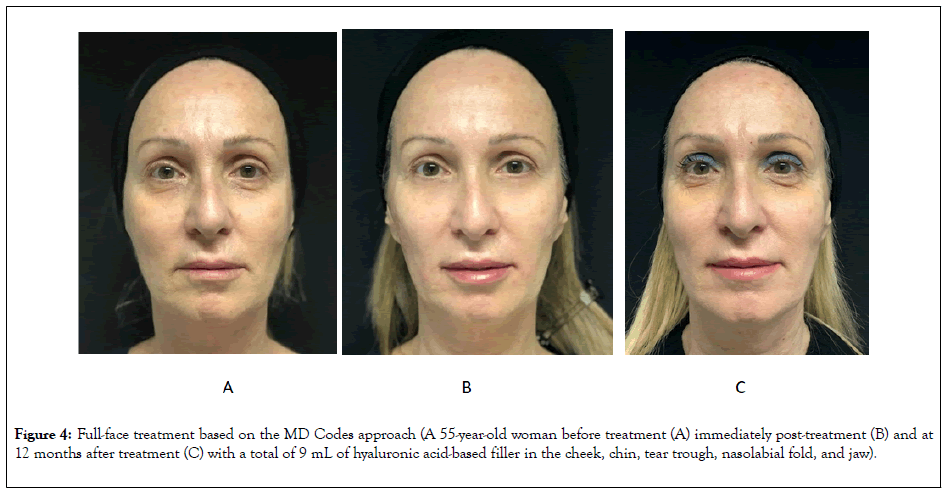
Figure 4: Full-face treatment based on the MD Codes approach (A 55-year-old woman before treatment (A) immediately post-treatment (B) and at 12 months after treatment (C) with a total of 9 mL of hyaluronic acid-based filler in the cheek, chin, tear trough, nasolabial fold, and jaw).
Complications
In total, 8 AEs were recorded in 6 patients (7.1%) (Table 2). There were 4 patients (4.8%) who reported bruising and 4 (4.8%) who reported pain. All AEs were minor and transient.
| Event | Patients, n (%) |
|---|---|
| Bruising | 4 (4.8) |
| Pain | 4 (4.8) |
Table 2: Adverse events.
Pain was related to trauma from the needle on the periosteum after the injection of Ck1 and resolved within 2 days; bruising resolved within 6 days. None of the AEs appeared to be related to the volume of filler used.
No serious AEs were reported, and there were no late-onset complications across the entire study period (up to 18 months).
This study demonstrates that full-face rejuvenation with HA fillers based on the MD Codes technique can be successfully performed without substantial safety concerns. A mean of 6.3 syringes of filler were used per patient. Almost 10 years ago, Taub and colleagues demonstrated in a small group of patients that higher volumes of HA fillers (6-8 mL) could be safely injected with perceived reductions in apparent age of 6-9 years [17]. The data from the present study add to the now substantial body of evidence showing that a full-face approach using HA fillers can be safe and efficacious, whether performed across a single visit or several [17-23].
The data also confirm that the MD Codes technique can be safely and efficaciously used in normal clinical practice. This approach is predicated on the assumption that a mean volume of HA filler of around 4 mL per session will be required to achieve significant clinical benefit. Although the MD Codes were developed for use with Vycross fillers, there is no theoretical reason why they could not be used with other products with similar underlying properties.
The MD Codes technique was designed to improve the standardization of treatment with HA fillers, optimize aesthetic outcomes, and help practitioners to avoid important anatomical structures, such as major blood vessels, nerves and glands. Erroneous injection of these areas may be associated with major complications, such as necrosis and blindness [24,25]. No patients in the present analysis experienced such events. Furthermore, study participants were followed for up to 18 months, with no evidence of delayed-onset complications, such as biofilm, nodules, foreign-body granuloma or scarring [26].
It is encouraging to note that the overall complication rate was only 7.1%; all of these cases were minor and transient issues relating to bruising and/or cheek injection-site pain. Some level of bruising and pain is to be expected with these procedures but can be minimized through reducing injection speed and avoiding aggressive fanning and large bolus sizes [24].
Interestingly, increased patient trust of their clinician also appears to reduce their perception of pain [27]. This highlights the importance of building a positive injector-patient relationship ahead of treatment. The use of MD Codes can be useful in this regard, allowing patients to get involved in treatment planning by pointing out specific subunits that are of most concern, and helping the injector to explain how the final treatment plan will address those concerns.
Although no objective patient- or injector-assessed measure of outcomes was made in the present study, informal feedback from patients suggested high levels of satisfaction. Treatment protocols were focused primarily on the midface: all patients received filler in the cheek, and the next two most commonly treated areas were the nasolabial fold and tear trough. The midface has been shown by computed tomography to undergo substantial age-related changes, including inferior migration of fat compartments and volume loss in the deep medial cheek fat [28]. Several studies have demonstrated that the fillers we used, particularly those with high G’ (VYC-20 and VYC-17.5), can durably correct the appearance of the midface and improve overall patient satisfaction [5,7,8,12,13]. Thus, injection of the cheek is a recommended element of full-face treatment with fillers and is also a logical place to begin such a treatment, because injection in the Ck1, Ck2 and Ck3 MD Codes typically produces an overall lifting effect, with indirect improvements often observed in other areas such as the nasolabial fold and tear trough.
In the current study, many patients were also treated in the lower face, particularly the chin, jawline and lip. Although the focus of treatment was primarily to achieve overall rejuvenation, some patients came with very specific concerns about particular areas of the lower face. Resolving these issues does not always mean injecting that specific area; sometimes, injection in another area of the face can indirectly produce the desired effect. For example, a recent case report showed how the chin reshaping concept from the MD Codes could be used to ameliorate naturally thick and everted lips in a young female patient [29].
We should acknowledge the limitations of the present study. Most importantly, it recruited patients from a single center and had a retrospective design. Nonetheless, the data reflect our experience and align with other studies of a full-face approach to treatment with HA-based facial fillers [17-23]. Prospective, multicenter, controlled studies assessing the utility of the MD Codes technique, as well as the relationship between injection volume and complications, would be welcome.
This study shows that full-face rejuvenation with HA fillers using the MD Codes technique can be undertaken successfully with minimal complications. The MD Codes may define a new standard of care in the use of these products.
Writing and editorial assistance was provided by Timothy Ryder, DPhil, of Biological Communications Limited (London, United Kingdom) and funded by Allergan plc (now Abbvie) at the request of the investigator. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Citation: Saliani MT (2020) Full-Face Rejuvenation with Hyaluronic Acid Fillers based on the MD Codes Technique: A Retrospective, Single- Center Study. J Clin Exp Dermatol Res. 11:523. DOI: 10.35248/2155-9554.20.11.523
Received: 26-May-2020 Accepted: 15-Jun-2020 Published: 22-Jun-2020 , DOI: 10.35248/2155-9554.20.11.523
Copyright: © 2020 Saliani MT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.