Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2013) Volume 3, Issue 6
Chemical composition and functional properties of legume seed flours and protein isolates prepared by isoelectric precipitation procedure (A) and micellization procedure (B) were investigated. Cowpea (Vigna ungiculata L.) Protein Isolates (CPIA, CPIB) and Lupin (Lupinus termis) Protein Isolate (LPIA, LPIB) were prepared from dehulled defatted seeds. Proximate analysis gave 26.73-43.57% protein, 2.30%-9.75% fat, 3.87%-3.16% total ash, 1.02%-12.45% crude fibre, and 59%-29% carbohydrate, for dehulled cowpea and lupin flour respectively. The protein percentage of isolates was found to be 75%-76% in (CPIA and CPIB) while 91%-87% for LPIA and LPIB, respectively. The minimum protein solubility for CPIA was at pH 5.0 and for CPIB at pH 4.0. Protein solubility values of LPIB were higher than that of LPIA. Protein isolates showed good solubility in all pH ranges. For water and oil absorption capacity, Dehullued Cowpea Flour (DCF) gave 1.30 ml water/g sample and 1.04 ml oil/g sample respectively; while CPIA 2.10 ml water/g sample and 1.93 ml oil/g sample, CPIB 2.33 ml water/g sample and 2.37 ml oil/g sample. Dehullued Lupin Flour (DLF) gave 1.80 ml water/g sample and 1.80 ml oil/g sample. However, LPIB had higher oil absorption capacity than LPIA. The highest Emulsifying Capacity (EC) was observed at pH 12.0 for Dehullued Defatted Cowpea Flour (DDCF) (173 oil/g protein), for CPIA (160 oil/g protein) while CPIB showed highest EC (137 oil/g protein) at pH 2.0. The EC for both (CPIA and CPIB) was higher at pH 7.0 compared to value obtained from DDCF. In fact, LPIB had higher fat absorption and emulsification capacity than LPIA. The least gelation concentrations for studied legume flours and protein isolates were noted at 12.0% (w/v).
Keywords: Cowpea; Lupin; Protein isolates; Functional properties
Legumes represent, together with cereals, the main plant source of proteins in human diet. They are also generally rich in dietary fibre and carbohydrates [1]. Minor compounds of legumes are lipids, polyphenols, and bioactive peptides [2]. Legumes will therefore continue to play important part in diets in the foreseeable future. Legumes provide a good source of protein (18-35%), supplement cereals not only for protein but also for minerals and vitamins of B complex. This is particularly important when refined cereals such as white wheat flour are used in a poor diet with few supplementary foods. Plant food diets increase the level of fibre intake which reduces the risk of bowel diseases, including cancer and also reduction in osteoporosis incidence [3]. High protein (18-35%) and carbohydrates (50-60%) contents together with amino acid pattern complementary to that of cereal grains; however make cowpea a potentially important nutritional component in the human diet ( [4]. Cowpea (Vignaungiculata L.) provides more than half the plant protein in human diets (Rachie, 1885). It is a good source of calories, vitamins and minerals and provides a significant amount of dietary protein and lysine. In regions of chronic protein shortage, it provides food of fairly high nutritive value to both humans and domestic animals [5]. However their protein digestibility is limited due to protein structure and also some antinutritional factors contributing to the poor protein quality include poor digestibility, deficient of sulfur amino acids and present of anti-nutritional factors (phytate, polphenols), enzyme inhibitors (trypsin, chymotrypsin) [6]. However the in vitro protein digestibility was very high in cowpea seeds (75.04-78.76%) [7].
Legume seeds are an abundant source of proteins and, among them; lupin is one of the richest. Lupin seed deserves great interest due to its chemical composition and augmented availability in many countries in recent years [8]. It is rated as being among eight potential sources of plant protein for the production of feeds and foods [9]. Lupin is a good source of nutrients, not only proteins but also lipids, dietary fibre, minerals, and vitamins [10-12]. In contrast to other leguminous plants, lupine seed contains more crude fibre, a proportion of which is viewed as dietetically beneficial [13].
In order to successfully introduce a new supplementation into any food item, it is imperative to find out if the supplementation possesses suitable functional properties for food applications and consumer acceptability. These functional properties are the intrinsic physico-chemical characteristics which may affect the behavior of food systems during processing, storage and consumption, such as solubility, foamability, gelation and emulsification properties [14]. Solubility of a protein is one of the critical functional attributes required for its use as a food ingredient, because solubility greatly influences other properties, such as emulsification, gelation and foaming [15]. Thus it determines the behavior of a protein food product. For plant proteins to be useful and successful in food application they should ideally possess several desirable characteristics, referred to as functional properties. Therefore, the present study was aimed to study the chemical composition and functional properties of the dehulled cowpea, bitter lupin seed flour and protein isolates by isoelectric and micelization.
Samples
Cowpea (Vigna unguiculata L.) and lupin (Lupinus termis) seeds (Figure 1) were brought from local market at Wad Medani city, Sudan and stored in polyethylene bags at room temperature (29-30°C).
Preparation of seed flours
The dehulled cowpea seeds and bitter lupin seeds were ground to pass through a 35 mesh. The flour was defatted by soaking in petroleum ether (BP. 40-60°C) at room temperature for 48 h with several changes of the solvent. The latter decanted and defatted flour was air dried over night at room temperature and kept in clean bottles, ready for analysis.
Preparation of protein isolate (A) by isoelectric precipitation
Protein isolates-A, Cowpea Protein Isolate (CPIA) and Lupin Protein Isolate (LPIA) were prepared from seed flours as shown in Figure 1 following the method described by Thompson [16] slightly modified by Mccurdy and Knipfel and Fernandez et al. [17,18]. The defatted flours were dispersed in distilled water in a 1:5 (w/v) ratio and the suspension adjusted to pH 9.0 using 1 N NaOH. The mixture stirred at room temperature for 20 minutes and insoluble matrices were separated by refrigerated centrifuge at 4000 g/20 min and discarded. Extraction and centrifugation procedures were repeated on the residue. The supernatant was adjusted to pH 4.0 using 1.0 N HCl and stirred at room temperature for 20 min. The mixture was centrifuged in a refrigerated centrifuge (4000 g/20 min). Precipitate was then washed several times, by distilled water until it was free from salt, and then neutralized to pH 7.0, using 1.0 N NaOH. The neutralized precipitate was left over night in refrigerator (4°C). This isolate was freeze-dried, ground into powder using a ceramic mortar and pestle and stored in a desiccators at room temperature ready for analysis.
Preparation of protein isolate (B) using micellization precipitation
Protein isolates-B, Cowpea Protein Isolate (CPIB) and Lupin Protein Isolate (LPIB) were prepared from seed flours as shown in Figure 2 and described by Lampart-Szczapa [19]. Defatted seed flour was suspended in NaCl 1.0 N solution in a 1:10 (w/v) ratio and stirred for 2 hr at room temperature. The suspension was centrifuged at 3000 g/30 min. and residue was extracted again as described above. The combined supernatant was diluted ten folds by distilled water and left to stand at refrigeration temperature (4°C) for 18 hr.
Chemical analysis: Cowpea and lupin seed flours and protein isolate composition were determined following methodology for total nitrogen (Kjeldahl), fat (Soxhlet), carbohydrates, moisture and ash (gravimetrically) and crude fibre by a chemical-gravimetric method AOAC [20], and the means reported on dry weight basis.
Functional properties
Protein solubility: The solubility of dehulled cowpea and lupin seed flour and protein isolates as a function of pH was determined using the method described by. The pH was checked and adjusted then centrifuged at 4000 rpm for 20 min at room temp, and the nitrogen in the supernatant or in aliquot (2.0 ml) of the clear supernatant was estimated by the Micro kjeldahl Method [21].
Water and oil absorption capacity: Water and oil absorption capacity of isolates was determined according to the method described by Beuchat [22].
The water and oil absorption capacity were calculated as follows:

Emulsifying capacity: Effect of pH on the emulsifying capacity: Emulsifying capacity was determined according to the procedure of Emulsification capacity was also determined in the pH range of 1-12 (2, 4, 7, 9 and 12) using 1 N HCl or I N NaOH solution.
Emulsifying capacity was calculated as follows:
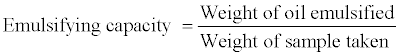
Where:
Weight of oil emulsified=Total volume of oil emulsified×specific gravity of oil used.
Gelation capacity
Least gelation concentration was determined using the method of Coffmann and Garciaj [23].
Results of the proximate composition of the seed flour and the protein isolates are presented in Table 1. The whole (WCF) and dehulled defatted (DDCF) cowpea seed flour contained 22.30%-26.73% protein, 2.10%-2.30% fat, 4.10%-1.02% fibre, 3.77%-3.87% ash and 60%-59% carbohydrates respectively (on dry weight basis) as major components. The data obtained is comparable to that reported by Sosulski et al., Abdalla et al. and Ragab et al. [5,24,25].
| Chemical constituents | WCF | DCF | BLF | CPIA | CPIB | LPIA | LPIB |
|---|---|---|---|---|---|---|---|
| Crude protein (N x 6.25) | 22.30 | 26.73 | 43.57 | 75.00 | 76.00 | 97.02 | 96.80 |
| Crude fat | 2.10 | 2.30 | 9.75 | Trace | Trace | Trace | Trace |
| Crude fibre | 4.10 | 1.02 | 12.45 | Trace | Trace | 0.80 | 1.05 |
| Total ash | 3.77 | 3.87 | 3.16 | 2.63 | 2.30 | 1.26 | 1.08 |
| Carbohydrate (by difference) | 60.07 | 59.78 | 29.00 | 13.00 | 13.10 | 3.30 | 3.75 |
Table 1: Proximate composition of dehulled cowpea and Bitter lupin seed flour and protein isolates.
Protein isolates (CPIA and CPIB) showed 75% and 76% protein content and a decrease in carbohydrate content from 59.78% to 13%. Bitter lupin protein isolate (LPIA and LPIB) contained 97.02%-96.80% protein, 0.15%-0.18% lipids, 0.80%-1.05% crude fibre, 1.26%-1.05% ash respectively (on dry weight basis). This finding may focus the interest of utilizing lupin seed flour as a high protein source in some food formulation. The obtained results agree well with those reported by Narayana and Narsing Rao, Chau and Cheung and Sathe et al. [26-28].
Protein solubility: The effect of pH on protein solubility
The protein solubility of cowpea and lupin seed flour and protein isolates at pH values ranging from 2-12. Is shown in Figure 3. The solubility behavior of cowpea and lupin seed flours gave a U-shaped curve in the pH 2-9 which is similar to many oil seed and most vegetable legume proteins [29]. Minimum solubility of DCF and CPIA were found at pH 5.0 while BLF and CPIB showed minimum solubility at pH 4.0.
Similar isoelectric point was observed in some legume flours such as lablab and cowpea flours [30] and check pea flour [31]. A protein in an aqueous system has a zero net charge at its isoelectric point and no migration occurs. At pH values above and below the isoelectric point the protein solubility progressively increased. Protein has a positive or negative charge at pH values above and below the isoelectric point, where more water increase with protein charges. Protein isolate studied showed good solubility in both acid and alkaline pH regions which is most important characteristics for food formulation [32]. Seed protein could find good applications in soft drinks and slightly acidic beverages to increase and improve its protein content and nutritional quality. Conformational features of proteins caused these differences in water absorption, also some other chemical compounds rather than protein particularly starch and crude fibre may take place in water binding capacity [33].
The results of water and oil absorption capacities of cowpea protein isolates-CPIA and CPIB (Table 2). were similar to that reported by Ragab et al. [25].The data obtained within the commercial values reported for protein concentrate (1.90-2.210 ml water/g protein) as reported by Lin and Zayas [34]. However, water absorption capacity of lupin protein isolates LPIA and LPIB were higher than that reported by Sathe et al. [35]. Generally the water absorption capacities of both isolates were higher than the values obtained from their flours. The low water absorption capacity of the flour may be attributes to its low protein contents. The results may give an advantage to cowpea and lupin isolates in some foods, especially comminuted meal with good water absorption capacity. Both CPIB and LPIB give higher values of fat absorption capacity (2.37 and 2.90 ml/g) respectively than isolates CPIA and LPIA (1.90 and 2.80 ml/g) respectively.
| Type of products | Water absorption capacity (ml/gm) | Fat absorption capacity (ml/gm) |
|---|---|---|
| Dehulled cowpea flour (DCF) | 1.30 | 1.04 |
| Lupin seed flour(LSF) | 1.34 | 1.76 |
| Cowpea protein isolate-A (CPIA) | 2.10 | 1.90 |
| Lupin protein isolate-A (LPIA) | 2.09 | 2.80 |
| Cowpea protein isolate-B (CPIB) | 2.33 | 2.37 |
| Lupin protein isolate-B (LPIB) | 2.21 | 2.90 |
Table 2: Water and fat absorption capacity of lupin, cowpea seed flours and protein isolates.
Variation in oil absorption capacity might be due to the different proportion of non polar side chains of the amino acids on the surface of the protein molecules. Several authors have reported the oil absorption capacity to non polar side chains of the protein as well as to different conformational features of the protein.
Emulsifying capacity (EC): Effect of pH on emulsifying capacity
The emulsifying capacity of lupin and cowpea seed flours over the pH range (2-12) is presented in Table 3. The emulsifying capacity vs pH profile of lupin seed flours showed a similar pattern to protein solubility vs pH profile suggestion that emulsifying property is mainly due to the protein solubility. The emulsifying capacity vs pH profile of lupin seed flour was similar to those reported by Crenwelge et al. [36] soy bean, Naryana and Narsing Rao [26] for winged bean. The emulsion capacity for both cowpea protein isolates (CPIA and CPIB) in the present investigation was higher, comparable to values reported for lupin seed protein concentrate (88.9). Sathe et al. [28], reported the values of several oil seed flours and protein concentrates/isolates [36,37]. The high emulsifying capacity of cowpea and lupin seed protein isolate may be useful for food applications. The emulsification capacity values for cowpea and lupin seed flour in alkaline pH was higher than in acidic pH; Results revealed that an alkaline pH improved the emulsifying capacity more than did the acidic pH. Emulsifying capacity was pH dependent; Emulsifying capacity was pH dependent, upon the hydrophilic-lipophilic balance [28,38], which was affected by pH.
| Type of product | pH | Emulsifying capacity g oil/g sample | Type of product | pH | Emulsifying capacity g oil/g sample |
|---|---|---|---|---|---|
| Lupin flour (LF) | 2 | 70.20 | Dehulled cowpea flour (DCF) | 2 | 105.80 |
| 4 | 40.56 | 4 | 133.70 | ||
| 6 | 82.65 | 6 | 73.86 | ||
| 8 | 110.80 | 8 | 72.73 | ||
| 12 | 125.20 | 12 | 173.60 | ||
| Lupin protein isolate-A (LPIA) | Cowpea protein isolate-A (CPIA) | ||||
| 2 | 164.00 | 2 | 133.60 | ||
| 4 | 140.32 | 4 | 107.40 | ||
| 6 | 108.55 | 6 | 133.90 | ||
| 8 | 120.00 | 8 | 146.90 | ||
| 12 | 125.42 | 12 | 160.60 | ||
| Lupin protein isolate-B (LPIB) | Cowpea protein isolate-B (CPIB) | ||||
| 2 | 196.02 | 2 | 137.70 | ||
| 4 | 176.00 | 4 | 111.10 | ||
| 6 | 110.33 | 6 | 106.80 | ||
| 8 | 130.00 | 8 | 115.50 | ||
| 12 | 180.04 | 12 | 120.20 |
Table 3: Effect of pH on emulsifying capacity of dehulled cowpea flour (DCF), lupin seed flour (LF) and protein isolates (CPIA, CPIB, LPIA and LPIB).
Similar observations on the pH dependence on emulsifying ability of proteins have been reported by several investigators [26,36,39-41]. Cowpea and lupin protein isolate-A (CPIA and LPIA) had lower emulsifying capacity than their isolates-B (CPIB and LPIB). Addition of salt may increased the EC of proteins, due to the fact that addition of salt improved the protein solubility, and therefore improved the emulsifying capacity. Differences were observed in the EC of both lupin and cowpea protein isolates. These differences might be due to the variations of the hydrophilic-lipophilic balance of the protein along the pH gradient (2-12).
Gelation
Gelation is an aggregation of denatured molecules. The result of gel formation of lupin and cowpea seed flour and protein isolates are shown in Table 4. The results showed that both lupin and cowpea seed flours were not able to form gel at lower concentration however, gelation was noted for both flour and protein isolates samples at 12% (w/v). These values are identical to those reported for cowpea protein isolate [42], lupin seed flour [28], (Phaseolus calcarus) [27] black gram flour [35] but lower than those reported for raw cowpea (16% W/V) [43] and (18% W/V) for raw cowpea flour as reported by Elkhalifa [30]. Sathe et al. [28] reported that the least gelation concentrations of the lupin seed flour and the protein concentrate were 14 and 18% (w/v) respectively. The variation in gelling properties of different legume flours may be due to variation in the relative ratios of different constituents such as proteins, lipids and carbohydrates make up of the legumes. Cowpea and lupin protein isolate-B (CPIB and LPIB) showed better gelation properties compared to protein isolate-A (CPIA and LPIA). Gelation capacities are interrelated to water absorption capacity, the low water absorption of (CPIA and LPIA) could explain the deficient gelation formation capacity.
| Materials | Percentage protein concentrate | ||||||
| 6 | 8 | 10 | 12 | 14 | 18 | 20 | |
| Dehulled cowpea seed flour (DCF) | - | - | - | + | + | + | + |
| Bitter lupin seed flour (BLF) | - | - | - | + | + | + | + |
| Cowpea protein isolate-A (CPIA) | - | - | - | + | + | + | + |
| Cowpea protein isolate-B (CPIB) | - | - | - | + | + | + | + |
| Lupin protein isolate-A (LPIA) | - | - | - | + | + | + | + |
| Lupin protein isolate-B (LPIB) | - | - | - | + | + | + | + |
Table 4: Least gelation concentrations of dehulled cowpea and lupin seed flours and protein isolates.
Lupin protein isolates A and B, (LPIA, LPIB) had higher fat absorption cpacity compared to cowpea protein isolates A and B, (CPIA, CPIB). In addition, LPIB showed higher fat absorption capacity than LPIA. However, least gelation capacity for cowpea and lupin flours and both types of protein isolates were observed at 12.0% (w/v).