Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2015) Volume 5, Issue 5
Novel calix[4]arene derivative containing mixed pendant arms in its lower rim, 25, 27- bis (diethylamino) ethoxy- 26, 28- (bis-methoxyethoxy) calix[4]arene, L3, has been synthesized to target the heavy metal cations. 1H NMR investigations seems to indicate that the receptor L3 interacts preferentially with Hg2+, Cd2+, Pb2+ and Ag+ in acetonitrile. Complexation studies in acetonitrile show that the lower rim groups of the receptor L3 are the active sites of its interaction with metal ions. These findings are corroborated by conductance measurements in acetonitrile, plots of molar conductance against the ligand/metal cation ratio reveal the formation of 1:1 complexes between this ligand with these cations. Standard thermodynamics parameters of complexation (log Ks, ΔHᵒ c, ΔSᵒ c, ΔGᵒ c) of L3 with Hg2+, Cd2+, Pb2+ and Ag+ in acetonitrile were determined using the Nano ITC (isothermal titration calorimetry). For all the systems investigated, the complexation process between these metal cations and the receptor L3 was enthalpically controlled. The enthalpic and entropic contributions to the Gibbs energy associated with these processes are analysed.
<Keywords: Calixarene receptor; Heavy metal cations; 1H NMR investigations; Conductance measurements; Thermodynamics parameters of complexation
The environmental and biologically concern caused by the presence of the heavy metal cations encouraged the researchers to discover the possibilities of the calixarene derivative in dealing with these cations [1-10]. The heavy metals which are released continuously from industrial, agricultural to the environmental will cause great problems to the biological life [1-3]. These elements cause significant effects on humans in all over the world [11-13]. These highly toxic metals are known to cause health problems such as brain damage [14-16], nephropathy [17-19], sluggishness [20,21], hyperirritability [22], restlessness [23], infertility [24] and a number of diseases in early aged children [19,25]. The removal of these trace heavy metal ions are tested by number of methods i.e., membrane filtration, osmosis, precipitation, oxidation/ reduction, microbiological activity, ion exchange and extraction etc. [26-28].
The supramolecular chemistry has provided a much better solution to the search for molecular structures that can serve as building blocks for the production of sophisticated molecules by anchoring functional groups oriented in such a way that they delineate a suitable binding site. This was achieved with the development of macrocyclic molecules such as synthetic crown ethers, cryptands, spherands [29], natural cyclodextrins [30,31] and calixarenes [32,33].
The use of the calixarene compounds which has a high selectivity is one of the proposed methods. The design of calixarene-based receptors able to interact selectively with a given guest is a challenging area of research. The structure of the ҅cone҆ p-tert-butylcalix[4]arene is shown in Figure 1.
The most common forms of calixarenes are insoluble in water, due to their aromatic components. They also possess limited solubility in organic solvents, thereby making their purification and characterization a formidable task. Most forms are sufficiently soluble to allow spectral analyses in a limited number of common organic solvents however [34].
Calix[4]arene-based macrocycles have attracted much attention because of their unique molecular structure and simple single-pot synthesis in supramolecular chemistry [29,32,35,36]. These molecules are generally ortho-substituted calix[n]arenes capable of alkali, alkaline earth and heavy metal ion recognition [37-39].
It has been reported that ester function derived from calixarenes possess remarkable tendency to bind IA group cations with unique size selectivity [2,3,40] as well as with ions and molecules of biological and environmental relevance [26]. Recently, a number of studies have been explored on the subject of the liquid-liquid, extraction or complexation of targeted ions [41-45]. It has been explored earlier that a calixarene with diester and/or dinitrile functionalities at its lower rim (narrow rim) can selectively extracts lead/mercury from an aqueous to the organic phase in liquid-liquid extraction processes [46]. Solangi et al. have explored Pb2+ selective extraction behavior of ester derivatives of calix[n]arenes [47]. They observed that the hexaester derivative of calix[6]arene is more selective and efficient ionophore as compared to tetraester derivative of calix[4]arene for the extraction of Pb2+ from aqueous to the organic phase. However, an early attempt was made by Roundhill et al. [48] to extract the toxic metals with calix[4]arenes modified with sulfur containing functionalities. Compounds were found to be effective extractants for Hg2+. Arnaud-Neu et al. [49] used ionophores for complexation of Pb(II), Hg(II) and Cd(II) cations by replacing hard oxygen-based binding group with softer sulfur based binders. Calixarenes with thioamide functions in lower rim have demonstrated good efficiency in the selective extraction of Cd(II) and Pb(II) ions. Solvent extraction of heavy metals with macrocyclic ligands based on calix [4]arenas was also studied by Dung and Ludwig [50].
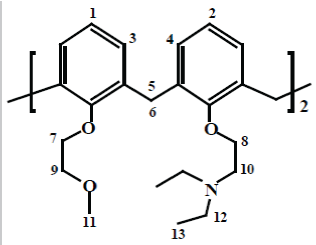
In this investigation, fully substituted, a novel calix [4] arene derivative containing mixed pendant arms in its lower rim, 25, 27-bis (diethylamino) ethoxy-26, 28-(bis-methoxyethoxy) calix[4]arene, L3, has been synthesized for the detection of heavy metal ions (Figure 2). This receptor was characterized by elemental analysis and 1H NMR and 13C NMR and 13C NMR-DEPT which confirmed that this receptor was successfully synthesized.
The capacity of a calixarene ligand to complex a specific guest species is determined by various structural factors, in particular, the type of donor groups and the shape of the cavity. A good response to cations was obtained by full functionalization of the lower rim with ether-amine groups. Calix(4)arene containing ether and amine functional groups at the lower rim have shown pronounced selectivity for metal cations in acetonitrile. It can be observed that the presence of the amino groups (basic) in the lower rim for this receptor makes this ligand attractive to explore their complexation with these metal cations.
Calixarenes are typical allosteric systems where changes on the one side lead to changes on the other side of macrocycle. The conformational changes that the calix [4] arene ligand undergoes upon complexation with ionic or neutral species can be assessed from 1H NMR investigations [51]. Gutsche et al. [52] established that the conformations of calixarenes and their derivatives can be established by observing the resonance of the methylene protons (axial and equatorial) in the 1H NMR spectrum. In the ̋ cone ̏ conformation Δδax-eq is about 0.90 ppm while values of 0.50 ppm and higher than 0.90 ppm are found for calixarenes in their flattened and distorted conformation respectively.
Investigation on the complexation of the L3 with metal cations in acetonitrile using a variety of techniques such as 1H NMR (to establish the binding sites and the conformational changes that these ligands undergo upon complexation with metal cations), conductance measurements (to establish the composition and the type and strength of the host-guest complexes). Nano ITC to derive the thermodynamics of complexation (stability constant, log Ks, hence standard Gibbs energy, ΔcG°; enthalpy, ΔcH°; and entropy, ΔcS° of complexation).
Chemicals
All chemicals were obtained from Sigma-Aldrich, Fluka and Fisher UK Scientific and were either analytical or reagent grade. Solid chemicals were used as received without further purification. All solvents were dried and purified as described in the literature [53]. Lead (II), cadmium (II), mercury (II), and silver tetrabutylammoniumperchlorate 99%, were dried over P4O10 under vacuum for several days before use.
1H NMR measurements
1H NMR measurements were used to characterize the calix[4] arene derivatives and to provide information about its interaction with heavy metals and whenever possible to establish the site of interaction of the ligand. 1H NMR measurements were recorded at 298 K on A Bruker DRX-500 pulse Fourier Transform NMR Spectrometer. The operating conditions involved pulse or flip angle of 30º, spectra width (SW) of 15 ppm, spectral frequency (SF) of 500.150 MHz, delay time of 0.3 s, acquisition time (AQ) of 3.17 s, and line broadening of 0.3 Hz. Solutions of the samples of interest (1 × 10-3 mol dm-3) were prepared in the appropriate deuterated solvent. These were placed in 5 mm NMR tubes using TMS (tetramethylsilane) as the internal reference.
Complexation studies: The complexation behavior of L3 toward metal cations at 298 K was studied using the 1H NMR technique, by adding the metal-ion salt (2.5 × 10-3 to 5.0 × 10-3 mol dm-3) into the NMR tube containing the ligand dissolved in the appropriate solvent (1.0 × 10-3 to 2.0 × 10-3 mol dm-3). Stepwise additions of the metal-ion salt were made and chemical shifts were recorded. Changes in chemical shifts upon addition of the metal-ion salt relative to the free ligand were calculated.
Conductance measurements
A Wayne-Kerr Autobalance Universal Bridge type B642 was used for conductometric measurements. The Wayne-Kerr is connected to a platinum glass bodied electrode housed in a cylindrical glass vessel where the reaction takes place. A thermostated bath circulating water in the vessel jacket was used to maintain the temperature of the vessel at 298.15 K. A magnetic stirrer was used to keep homogeneous the solutions throughout the time of the experiment. The conductance cell was a Russell type glass bodied electrode with a cell constant (determined using 0.10 mol dm-3 aqueous KCl solution). For these experiments, the vessel was filled with metal cations in the appropriate solvent (25 cm3) and the conductance of the solution was measured. Then, a known volume of solution of calix[4] arene derivative in the same solvent was added stepwise into the vessel and the conductance measured after each addition.
Determination of the constant of the conductivity cell: The cell constant was determined by titrating a solution of KCl (0.1 mol dm-3) in deionised water (25 cm3) [54]. The cell was immersed in a thermostated bath at 298.15 K. The conductance of the solution was recorded after addition of KCl once the stability of the system was ensured.
Nano ITC (Isothermal Titration Calorimetry)
ITC measurements were performed in a Nano Isothermal Titration Calorimeter, models 5300 (TA Instruments). All measurements were carried out in acetonitrile solvent at a fixed temperature of 298.15 K. The basic principle of ITC is simply to measure the heat released or absorbed in a liquid sample after the addition of another liquid sample. This heat is proportional to the total amount of binding that occurs within the calorimeter cell. The instrument has a pair of identical cells (1.4 ml), denoted as the reference and sample cells. These cells, along with access stems, are enclosed in a temperature-controlled thermal jacket. The reference cell was filled with acetonitrile. The ligand solution (1 m M) placed in the sample or reaction cell. The metal cations (20 m M) loaded in the syringe. The interval time between two readings was set at 240 s. The experiments were designed for a total of 25 consecutive injections. The power (or heat) difference between the sample and reference cells is used to determine reaction stoichiometry or number of binding sites (n), stability constant (Ka), and the enthalpy (ΔHº) [55,56]. The first data point was removed from the data set prior to curve fitting. The data was analyzed to determine the heat of interaction by using Origin 7.0 and Nano Analyzer data analysis software’s supplied by Microcal and TA Instruments, respectively with the ‘independent sites’ model.
Calibration of the equipment: To determine the accuracy of measurements carried out in the Nano ITC, a chemical calibration should be performed. The following equilibrium is established upon the addition of barium chlorideto 18-crown-6 ether in water at 298.15 K [57]. The sample cell was filled with an aqueous solution of 18-Crown-6 (1 × 10-3 mol dm-3) and titrated incrementally from the burette stirring system with BaCl2 (0.015 mol dm-3). The thermodynamic parameters for the complexation of Ba2+ with 18-C-6 in aqueous medium show a good agreement with the values reported in the literature by Briggner and Wadso (Appendix B).
Synthesis
Synthesis of 25, 26, 27, 28-tetrahydroxycalix[4]arene via de-tertbutylation of p-tert-butylcalix[4]arene(L1): The removal of p-tert-butyl groups from p-tert-butylcalix [4]arene was achieved by a catalyzed retro friedel-Crafts reaction as described by Gutsche et al. [58].
Synthesis of 25, 27 dihydroxy 26, 28- bis(diethylamine) ethoxycalix(4)arene, L2: The preparation of this derivative was achieved by procedure reported in the literature [59].
Synthesis of 25, 27-bis (diethylamino)ethoxy-26,28-(bismethoxyethoxy) calix[4]arene, L3: The 25, 27 dihydroxy 26, 28- bis(diethylamine) ethoxycalix(4)arene, L2 (1 g, 1.60 mmol), sodium hydride (1 g, 41.6 mmol) were suspended in 150 ml mixture of freshly refluxed THF and 30 ml of DMF dried on molecular sieves. Then 2-Bromoethylmethylether (0.64 g, 4.6 mmol) in DMF (10 ml) was syringed to the reaction mixture. Then the reaction was stirred and refluxed for 24 hr at 90°C. The reaction was monitored by TLC using DCM/Methanol (9:1) as developing solvent. After cooling down the reaction, the solvent was filtered through filter paper and removed under vacuum, to give an oily product which was dissolved by small amount acetonitrile followed by precipitation with drops of water afforded the pure compound as a white powder. Yield: 0.8 g, 1.08 mmol (67.80%). The compound was characterized by 1H-NMR in CD3CN at 298 K and microanalysis. 1H-NMR (CD3CN, 500 MHz) ; Ϩ(ppm)=6.81 (d, H-1); 6.67 (t, H-2); 6.60(d, H-3); 6.52(t, H-4); 4.51(d, H-6(ax)); 3.19(d, H-5(eq)); 4.17 (t, H-7); 3.98(t, H-8); 3.84(t, H-9); 2.95(t, H-10); 3.35(s, H-11); 2.60(q, H-12), 1.02(t, H-13). 13C NMR(CDCl3, 500 MHz) ; Ϩ(ppm)=122.15 (s, Ar, 5, 12, 19, 26), 127.80(s, Ar, 11, 13, 25, 27), 128.55(s, Ar, 4, 6, 18, 20), 134.01(s, Ar, 1, 10, 14, 24), 135.90 (s, Ar, 3, 7, 17, 21), 155.48 (s, Ar, 15, 28), 157.17 (s, Ar, 8, 9), 72.88(C38), 72.62(C35), 58.72(C29), 52.56(C30), 47.61(C31), 30.95(C37), 30.87(C2), 11.96(C32). 13C NMR-DEPT: Ϩ (ppm): 11.97 (C30), 30.97 (C31), 58.74 (C2), 122.17 (C29), 127.81 (C35), 128.56 (C36). Elemental analysis carried out, % found for C, 74. 81, N; 3.82 and H; 8.45, % calculated, C; 74.79, N; 3.79 and H, 8.40.
1H NMR characterization of the calix [4] arenes derivatives
The NMR spectra of these receptors (L1, L2 and L3) in CDCl3 at 298 K were recorded (Appendix A). The 1H NMR spectrum of L3 in CDCl3 shows that this ligand has thirteen different sets of protons. Five triplets at 4.16, 3.98, 3.84 and 1.01 ppm corresponding to proton 7, 8, 9, 10 and 13 respectively. The signal observed at 3.35 ppm is those corresponding to proton 11 that is found on the terminal ethyl group respectively. The pair of doublets at 4.51 and 3.19 ppm corresponds to the axial and equatorial protons respectively. The difference between these chemical shifts (Δδax-eq) was found to be 1.31 ppm. These results indicate that this macrocycle adopts a distorted ʻconeʼ conformation. This might be due to the steric and electrostatic effects between the pendent arms at the lower rim. Thus, these groups try to move away as possible from each other to reduce the steric and electrostatic effects. Two pairs of doublets were found at 6.80 and 6.60 ppm corresponding to the aromatic protons in meta position and a multiple peak (6.67 and 6.52 ppm) corresponding to the protons in para position. One quartet found at 2.59 ppm is assigned to the protons in the terminal ethyl chain.
13C NMR of L3 in CDCl3 at 298.15 K: 13C NMR spectrum of L3 in CDCl3 at 298.15 K is shown in (Appendix A).
1H NMR studies of L3 with tetrabutylammoniumperchlorate as counter ion in CD3CN at 298 K: It is very important at the start, and to be very exact, to examine the interaction between the counter ion (tetrabutylammoniumperchlorate) to these toxic metal cations with the receptor L3. It must be found out whether a receptor - counter complexes will be formed. 1H NMR spectrum of the receptor L3, with the counter ion (tetrabutylammoniumperchlorate) in acetonitrile at 298 K is shown in (Appendix A). It can be seen that no chemical shift changes are observed for L3 upon addition of the counter ion. It is indicate that the L3 receptor rejects the counter ion.
1H NMR studies on the interaction of L3 with metal cations in CD3CN at 298 K: 1H NMR spectra of the metal- receptor complexes at 298 K are shown in (Appendix A). The relevant 1H NMR chemical shift changes of the protons observed by the addition of heavy metal cations (as tetrabutylammoniumperchlorate) to L3, in CD3CN at 298 K are listed in Table 1.
| Receptor L3 | δ in CD3CN | L3 | L3 + Ag+ | L3 + Cd2+ | L3 + Hg2+ | L3 + Pb2+ |
|---|---|---|---|---|---|---|
 |
H-1 | 6.60 | - 0.39 | -0.07 | -0.12 | -0.09 |
| H-2 | 6.52 | - 0.22 | 0.02 | 0.02 | -0.01 | |
| H-3 | 6.81 | 0.31 | 0.11 | 0.15 | 0.1 | |
| H-4 | 6.67 | 0.26 | 0.1 | 0.15 | 0.09 | |
| H-5 | 3.19 | 0.05 | 0.11 | 0.12 | 0.1 | |
| H-6 | 4.51 | - 0.09 | -0.13 | -0.11 | -0.14 | |
| Δδ(ax-eq) | 1.32 | 1.18 | 1.08 | 1.09 | 1.08 | |
| H-7 | 4.16 | 0.16 | 0.02 | 0.04 | …… | |
| H-8 | 3.98 | 0.03 | -0.23 | 0.19 | …… | |
| H-9 | 3.84 | - 0.23 | -0.22 | …… | …… | |
| H-10 | 2.95 | 0.37 | …… | 0.81 | 0.79 | |
| H-11 | 3.35 | - 0.01 | -0.03 | -0.03 | -0.05 | |
| H-12 | 2.59 | 0.24 | 1.02 | 1.03 | 1.03 | |
| H-13 | 1.01 | 0.20 | 0.30 | 0.32 | 0.3 |
Table 1: 1H NMR chemical shift changes observed via the addition of metal cation salts to L3 relative to the free ligand (L3) in CD3CN at 298 K.
Table 1 shows the chemical shift changes of L3 induced by addition of heavy metal cation salts (as tetrabutylammoniumperchlorate) in CD3CN at 298 K. These changes suggest that interaction of L3 and these metal cations is taking place. It can be seen that Δδax-eq decreased from the free to the complexes ligand from 1.32 to 1.18, 1.07, 1.08, 1.09, 1.08 ppm for Ag+, Cd2+, Hg2+ and Pb2+ respectively, suggesting that the ligand adopts a “cone” conformation approximately upon complexation with these metal cations in this solvent.
Approximately, all protons for ligand L3 have been affected upon the addition of Ag+, Cd2+, Hg2+ and Pb2+ in CD3CN at
298 K. Some protons have shielding effects and others have deshielding effects. It can be noted that the protons closest to the nitrogen and the oxygen atoms such as H-7, H-8, H-9, H-10, H-11 H-12 and H-13 have considerable chemical shift changes relative to others. This is an indication that the lower rim groups of the receptors interact with these metals. Protons such as H-1, H-2, H-3, H-4, H-5 and H-6 have been also affected as a result of this interaction. The significant shift is observed for the methylene protons of the amine groups CH2NCH2CH3(H-10) which exhibit high upfield shifts of 0.37, overlap, 0.81, 0.79 ppm for Ag+, Cd2+, Hg2+ and Pb2+ respectively. These may indicate that the amine and possibly the ethereal oxygens are strongly involved in the complexation process, although the nitrogen atoms seem to interact more strongly. This upfield shift can be explained by assuming that the amine groups move inward the hydrophilic cavity toward the metal cation upon complexation which brings the CH2 protons toward the exterior of the hydrophilic cavity where they experience a shielding effect in the presence of the aromatic rings.
Conductometric measurements
With the aim of establishing the composition of the metal-ion complex in acetonitrile at 298 K. Conductometric curves for the titration of heavy metal cations with L3 in acetonitrile at 298 K are shown in Figure 3. These are plots of molar conductances (Ʌm, S cm2 mol-1) at each step of the titration against the [L3]: [Mn+] molar ratio.
Among the cations investigated, strong complexes of 1:1 stoichiometry were found for Ag+, Cd2+, Hg2+ and Pb2+. It can be seen that the conductomeric titration curves shows an decrease in molar conductance of the complexes throughout the titration, until the ligand/ Mn+ concentration ratio reaches 1:1. Then the molar conductance remains almost constant until the end of the experiment. This is due to the larger size of the complex relative to that of the free cation. Therefore, the mobility of the cation decreases leading to a decrease in the molar conductivity. Sharp break points are observed for Cd2+, Hg2+ and Pb2+ and L3 in this solvent at 1: 1 (ligand:metal cation) molar ratio suggesting that relatively strong complexes are formed in acetonitrile between this ligand and these cations. These findings are in agreement with 1H NMR measurements where significant chemical shift changes were observed by the addition of heavy cations (Cd2+, Hg2+ and Pb2+) to the ligand in CD3CN.
Thermodynamics of complexation
Standard thermodynamics parameters of complexation (log Ks, ΔHᵒc, ΔSᵒc, ΔGᵒ c) of L3 with heavy metal cations in acetonitrile were determined using the Nano ITC. For this purpose the instruments were electrically and chemically calibrated prior to measurements. ITC has been used to quantify the affinity of compounds for metal cations. Sessler et al. reported that crown-6 calix[4]arene-capped calix[4] pyrrole contained both cation and anion-recognizing sites and was an ion-pair receptor for CsF through ITC and 1H NMR investigations [60]. Calorimetric titration curves for the titration of metal cations with the receptor L3 in acetonitrile at 298 K were recorded (Appendix B).
Thermodynamic parameters of complexation of L3 with heavy metal cations in acetonitrile at 298.15 K: Titration calorimetry was used to obtain the log Ks and the enthalpy of complexation of L3 with cations in acetonitrile. Combination of Gibbs energies and enthalpies led to the calculation of the entropies associated to the complexation process. Thermodynamic data for the complexation of L3 with the cations in acetonitrile are summarized in Table 2.
| Complexes | Log K | ∆cHᵒ(kJ. mol-1) | ∆cGᵒ(kJ. mol-1) | ∆cSᵒ(J. mol-1.K1) | n |
|---|---|---|---|---|---|
| L3 + Ag+ | 4.80 | -35.71 | -27.40 | -27.87 | 1.01 |
| L3 + Cd2+ | 5.73 | -43.88 | -32.71 | -37.46 | 1.04 |
| L3 + Hg2+ | 6.50 | -60.52 | -37.10 | -78.55 | 1.03 |
| L3 + Pd2+ | 6.01 | -51.43 | -34.30 | -57.62 | 0.93 |
Table 2: Thermodynamics of complexation of L3 and metal cations in acetonitrile at 298.15 K.
For this ligand and these metal cations complexation, the stoichiometry n value was equal to 1, an ideal 1:1 metal ligand complex was formed. These findings are corroborated by conductance measurements in acetonitrile, plots of molar conductance against the ligand/metal cation ratio reveal the formation of 1:1 complexes between this ligand with these cations.
general analysis of the thermodynamic parameters shows that the complexation process is favored in terms of enthalpy (ΔHᵒ<0) but not in terms of entropy (ΔSᵒ<0) in all the above systems. Therefore, the complexation process is enthalpically controlled.
From the above discussion on the calix[4]arene derivative, the following conclusion can be drawn. The ligand under investigation (L3) were successfully synthesised in good yields and characterized by 1H NMR. From 1H NMR studies, it is concluded that L3 interact with Ag+, Cd2+, Hg2+ and Pb2+. The presence of the amino groups (basic) in the lower rim for calix[4]arenes makes this ligand attractive for exploring their complexation with these cations. The 1H NMR techniques was successfully used for establishing the binding sites and the conformational changes that this ligand undergoes upon complexation with the metal cations. The results obtained seem to indicate that the sites of interaction of this ligand with the heavy metal cations are amine group and ethoxy group. Indeed significant chemical shift changes in the proton close to the amine group and ethoxy group were observed. Conductomeric measurements were carried out with the aim of determining the composition of the receptor - cation interaction and gaining information regarding the type and strength of interaction of this receptor with these cations in acetonitrile at 298 K. Nano isothermal titration calorimetry is the most powerful tool to determine the enthalpies of binding of various reactions, including cation - ligand binding. Isothermal titration calorimetry (ITC) provides the most accurate and direct measurement of the enthalpy of any reaction under isothermal and isobaric conditions. It is also the only method capable of determining the enthalpy, entropy, and the Gibbs free energy of a reaction in a single titration experiment. Future work will involve the attachment of the receptor to a solid support to generate recyclable materials for cations removal.
The author thanks the Iraqi Government, Ministry of Higher Education and Basrah University for the financial support provided for this work.