Short Communication - (2022)Volume 12, Issue 2
Fungal Mitigation of Sodium Chloride and Chloroform of Rivers and Canals
Solomon I. Ubani*
*Correspondence:
Dr.
Solomon I. Ubani, Department of Nature Sciences,
Gaiasce Company and Gss Subsidiary,18 Haymarket Street, Manchester,
United Kingdom,
Tel: 447405536727,
Email:
Author info »
Abstract
The aim was to research the stampering of growth of fungi infestation in small rivers and oceans. A common species of this organism is ectomycorrhizal found on fruit bodies. These were examined using emission microscopy. This was inhabited by fresh water creatures and lifeforms. The objective was to increase deman with sustainable alternatives. The fungi were considered as a parasite to wild life. Microscopy when the follicle diameter was ≥ 1.02 mm using chloroform in relation to sodium chloride. This improved the breeding of sea going creatures. The competitors of these fungi were filamentous fungi which is the byproduct of depletion of organic matter of fungi growth. The sodium produced larvae of values of 1, 5, 10 and the chloroform acted as a reservoir of 2 and 3 growths. The time taken for addition of the treatment was a few days for spore production.
Keywords
Fungi; Sodium chloride; Chloroform; Microscopy
Introduction
The chloroform has co-occurring properties. It ensures survival
of sea going in non-salty riverine. Larvae of insects are considered
competitors to filamentous fungi. These parasites counteract the
development. Sodium chloride knows as salt does not cover well
the hyphal fungi organisms [1-6]. These had a negative effect on
the number of larvae. Chloroform was more readily absorbed
by the filamentous fungi. Sodium chloride has a minor effect
on fungi growth with 1, 5 and 10 larvae reductions of growth.
Chloroform increased the larvae with a selective priority growth
of larvae. The rivers and canals during this research were observed
for 12 days to ascertain the performance of fungi treatment [7-12].
Materials and Methods
A pine-oak forest with rivers and canals passing on its shores,
this had a high biodiversity. This report was based on a field
study of 12 days. To understand the status of the remnant of
the sea going creatures [13-17]. The growth of fungi was analyzed
using spectrometer and microscopy. This was used for obtaining
topographic measurements. The objective was prevention of
desalination of the rivers and canals [18-24]. Mass spectroscopy
was used to obtain the challenges in the fungi reduction for larva
growth for seagoing creatures (Tables 1 and 2).
| Properties of sodium chloride |
| Composition |
NaCl |
| Density |
2.17 g/cubic cm |
| Melting limit |
801°C |
| Evaporation limit |
1413°C |
| Classification |
Salt |
Table 1: Properties of sodium chloride.
| Properties of chloroform |
| Composition |
CHCl |
| Density |
1.48 g/cubic cm |
| Melting limit |
63.50°C |
| Evaporation limit |
61.20°C |
| Classification |
Chloroform |
Table 2: Primary and secondary outcomes.
Assessment and measures
A bioreactor was used to store samples of rivers and canals for each treatment. This was designed for algae growth. The samples were
taken to ensure nutrients were not counteracting eutrophication
[25]. The number of representative samples was n≈1000 in as
many paths of the pine-oak forests.
Surface tension
The chloroform and sodium chloride were added to the samples
in the bioreactor. A microscope was used to observe the physical
activity. The survival rate was measured according to upper and
lower limit of 2-6 on a scale of 1 to 10. The quality of life between
7-12 on a scale of 1 to 20. When the survival rate was high the surface tension was low whereas when the quality of life was high
the surface tension was high. This was a quantitative assessment
of the reactions occurring in the bioreactor [26].
Pearson χ2 test
This was used to indicate a linear trend. To assess the statistical
significance of the measured results. The logistic models
indicated the survival rate and quality of life of the samples
after chloroform and sodium chloride treatment. These two
variables had more than two categories. The effect showed the
modifications and interactions for Probability values, P<0.05
were significant [27,28].
Fungi density
The percentage of sediments in the samples in the bioreactor was
used to ascertain the depletion of fungal growth. The bulk density
in chloroform pretreatment was normal between 20-25 kg/m2.
The density of sodium chloride was low less than 30 kg/m2 [29- 32]. The interaction between these three factors were analysed
and associations were considered non-significant at P=0.15.
Results
The results of the multivariate analysis were shown in Table
3 for both sodium chloride and chloroform treatment. The
survival rate was written as a ratio. For sodium chloride it was
1.53 and for chloroform it was 1.72. The quality of life was
written as a ration for sodium chloride it was between 1.22-1.93
and chloroform it was between 1.39-2.14. These were the pre-
treatment values [33-38]. The post-treatment yielded different
values due to disassociation of the fungal growth. The survival
rate for sodium chloride was 1.36 and quality of life was 1.03-
1.80. The chloroform survival rate was 1.90 and quality of life
was 1.63-2.22. Thus was statistically higher for the larvae growth
[39-41].
|
Sodium chloride |
Chloroform |
| Survival rate |
Quality of life |
Fungal density |
Survival rate |
Qaulity of life |
Fungal density |
| Nominal |
Min |
Max |
Nominal |
Min |
Max |
Nominal |
Min |
Max |
Nominal |
Min |
Max |
| Pre-treatment |
1.53 |
1.22 |
1.93 |
1.53 |
1.25 |
1.88 |
1.72 |
1.39 |
2.14 |
1.64 |
1.31 |
2.04 |
| Post-treatment |
1.36 |
1.03 |
1.8 |
1.71 |
1.37 |
2.13 |
1.9 |
1.63 |
2.22 |
1.61 |
1.39 |
1.86 |
Table 3: Statistical significance of sodium chloride and chloroform treatment.
The fungal density for sodium chloride pretreatment was
nominal 1.53 and posttreatment nominal was 1.71. The fungal density changed during the 12 day time for sodium chloride
was between 1.25 -1.88 for pre-treatment and 1.37-2.13 for post-
treatment. For chloroform was between the chloroform density
nominal was pretreatment was 1.64 and post treatment 1.61 of
the bioreactor. The fungal density changed during the 12 day
time for pre-treatment was between 1.31-2.04 and posttreatment
between 1.39 -1.86 [41-45].
Discussion
The purpose of the study was to estimate quantitatively the
prevalence of fungi growth using the treatments. This was a
survey of a pretreatment initial process and a comprehensive
posttreatment of the rivers and canals. The previous research
estimated the activity had not been representative of population
of an entire geographical region due to local approach [46- 49]. The population of the treatment had sample averages and
unequal variances. The pearson χ2 test was used to evaluate the
depth of fungal depletion before replenishment (Table 4).
| t-test: two-sample assuming unequal variances |
Sodium Chloride |
Chloroform |
| Mean |
1.556666667 |
1.706666667 |
| Variance |
0.090546667 |
0.112226667 |
| Observations |
6 |
6 |
| Hypothesized mean difference |
0 |
|
| df |
10 |
|
| t stat |
-0.815946087 |
|
| P(T<=t) one-tail |
0.21676825 |
|
| t critical one-tail |
1.812461123 |
|
| P(T<=t) two-tail |
0.433536499 |
|
| t critical two-tail |
2.228138852 |
|
Table 4: Pearson χ2 test of the post-treatment
There was a prevalence of the sedimentation of the sodium
chloride for fungal growth initially. This had a percentage effect
between 54.5 to 71% and the chloroform had a greater difference
between 43.3 to 87.8% [49-52] (Figure 1).
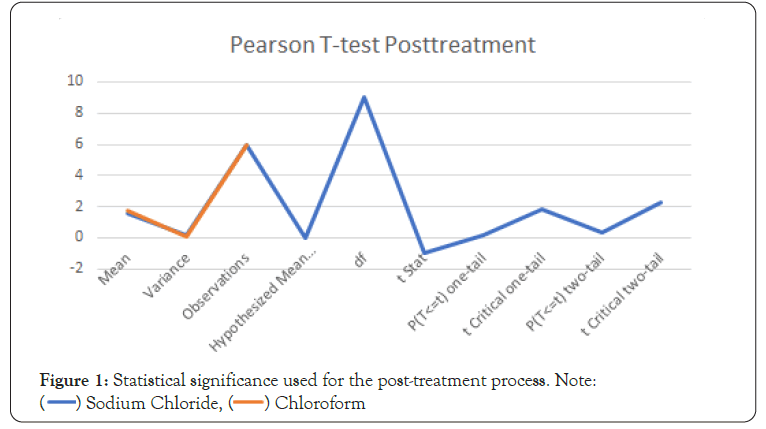
Figure 1: Statistical significance used for the post-treatment process. Note: 
The statistical significance graph shows the first 6 days both
the sodium chloride and chloroform performed well in fungi
treatment. After this the chloroform had a more lasting effect
with the greatest 9 days from treatment (Table 5).
| t-test: two-sample assuming unequal variances |
Sodium chloride |
Chloroform |
| Mean |
1.556666667 |
1.706666667 |
| Variance |
0.090546667 |
0.112226667 |
| Observations |
6 |
6 |
| Hypothesized mean difference |
0 |
|
| df |
10 |
|
| t stat |
-0.815946087 |
|
| P(T<=t) one-tail |
0.21676825 |
|
| t Critical one-tail |
1.812461123 |
|
| P(T<=t) two-tail |
0.433536499 |
|
| t critical two-tail |
2.228138852 |
|
Table 5: Pearson χ2 test of the pre-treatment
The activity was about 23% increase from the pretreatment of the
fungal growth [52-58]. The application of the measures to estimate
the activity obtained a large difference in sodium chloride and
chloroform of the bioreactor samples (Figure 2).
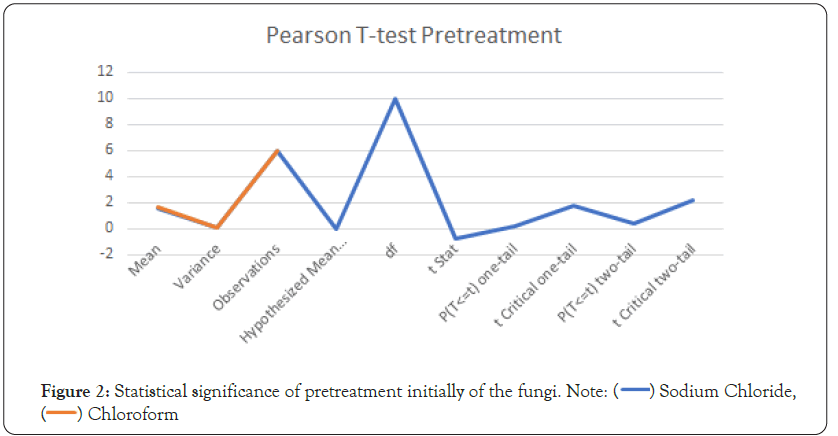
Figure 2: Statistical significance of pretreatment initially of the fungi. Note: 
The degree of change for the sodium chloride for larvae growth
was 6 whereas for chloroform it was 8. Therefore the depletion
used in activities involved larvae ≥ 2. The reactions were not
observed until this value [58-65].
Conclusion
It can be concluded the prevalence of fungi was especially
high among the sodium chloride treatment. The chloroform
showed similar trends in the first 6 days. This was used to
assess the prevalence. The P-vales for the Pearson χ2 had a linear trend 0.21676825- 1.812461123; **P=0.43353649-
2.22813885;***P<0.5. Therefore, the results were significant for
the research. This project was financially supported by Gaiasce
Company and Gss subsidiary.
References
- Adams CA, Andrews JE, Jickells T. Nitrous oxide and methane fluxes vs. carbon, nitrogen and phosphorous burial in new intertidal and saltmarsh sediments. Sci Total Environ. 2012;434:240-51.
[Crossref] [Google Scholar]
- Allen JR, Duffy MJ. Medium-term sedimentation on high intertidal mudflats and salt marshes in the Severn Estuary, SW Britain: the role of wind and tide. Mar Geol. 1998;150(1-4):1-27.
[Crossref] [Google Scholar]
- Allen JR, Duffy MJ. Temporal and spatial depositional patterns in the Severn Estuary, southwestern Britain: intertidal studies at spring–neap and seasonal scales, 1991–1993. Mar Geol. 1998;146(1-4):147-171.
[Cross ref] [Google Scholar]
- Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81(2):169-193.
[Crossref] [Google Scholar]
- Bischoff J, Sparkes RB, Doğrul Selver A, Spencer RG, Gustafsson Ö, Semiletov IP, et al. Source, transport and fate of soil organic matter inferred from microbial biomarker lipids on the East Siberian Arctic Shelf. Biogeosciences. 2016;13(17):4899-4914.
[Crossref] [Google Scholar]
- Blackwell MS, Yamulki S, Bol R. Nitrous oxide production and denitrification rates in estuarine intertidal saltmarsh and managed realignment zones. Estuar Coast Shelf Sci. 2010;87(4):591-600.
[Crossref] [Google Scholar]
- Chen J, Wang D, Li Y, Yu Z, Chen S, Hou X, et al. The carbon stock and sequestration rate in tidal flats from coastal China. Global Biogeochem Cycles. 2020;34(11):e2020GB006772.
[Crossref] [Google Scholar]
- Bradfer‐Lawrence T, Finch T, Bradbury RB, Buchanan GM, Midgley A, Field RH. The potential contribution of terrestrial nature‐based solutions to a national ‘net zero’climate target. J Appl Ecol. 2021;58(11):2349-60.
[Crossref] [Google Scholar]
- Pontee N. Accounting for siltation in the design of intertidal creation schemes. Ocean and coastal management. 2014;88:8-12.
[Crossref] [Google Scholar]
- Brown SL, Pinder A, Scott L, Bass J, Rispin E, Brown S, et al. Wash Banks Flood Defence Scheme Freiston Environmental Monitoring 2002-2006. Report to Environment Agency, Peterborough. Centre for Ecology and Hydrology, Dorset, UK UK. 2007.
- Garbutt A. Bed level change within the Tollesbury managed realignment site, Blackwater estuary, Essex, UK between 1995 and 2007. NERC Environmental Information Data Centre. 2018.
[Crossref] [Google Scholar]
- Bull JW, Milner‐Gulland EJ. Choosing prevention or cure when mitigating biodiversity loss: Trade‐offs under ‘no net loss’ policies. J Appl Ecol. 2020;57(2):354-366.
[Crossref] [Google Scholar]
- Burden A, Garbutt A, Evans CD. Effect of restoration on saltmarsh carbon accumulation in Eastern England. Biology letters. 2019;15(1):20180773.
[Crossref] [Google Scholar]
- Burden A, Garbutt RA, Evans CD, Jones DL, Cooper DM. Carbon sequestration and biogeochemical cycling in a saltmarsh subject to coastal managed realignment. Estuar Coast Shelf Sci. 2013;120:12-20.
[Crossref] [Google Scholar]
- Jacobs. Final Far Field Effect and Channel Exit: Review and summary –Note 6. Report prepared 655 for the Environment Agency by Jacobs, uk. 2019:39.
- Duarte CM, Dennison WC, Orth RJ, Carruthers TJ. The charisma of coastal ecosystems: addressing the imbalance. Estuaries Coast. 2008;31(2):233-238.
[Crossref] [Google Scholar]
- Emmer I, Needelman B, Emmett-Mattox S, Crooks S, Megonigal P, Myers D, et al. VM0033 637Methodology for tidal wetland and seagrass restoration. Version 1.0. Verra. Verified Carbon 638 Standard, 2015.
- Friedlingstein P, O'sullivan M, Jones MW, Andrew RM, Hauck J, Olsen A, et al. Global carbon budget 2020. Earth Syst Sci Data. 2020;12(4):3269-3340.
[Crossref]
- Gulliver A, Carnell PE, Trevathan-Tackett SM, Duarte de Paula Costa M, Masqué P, Macreadie PI. Estimating the potential blue carbon gains from tidal marsh rehabilitation: A case study from south eastern Australia. Front MarSci. 2020;7:403.
[Crossref] [Google Scholar]
- Guo LB, Gifford RM. Soil carbon stocks and land use change: a meta analysis. Glob Change Biol. 2002;8(4):345-360.
[Crossref] [Google Scholar]
- Hoogsteen MJ, Lantinga EA, Bakker EJ, Groot JC, Tittonell PA. Estimating soil organic carbon through loss on ignition: effects of ignition conditions and structural water loss. Eur J Soil Sci. 2015;66(2):320-328.
[Crossref] [Google Scholar]
- Daviet F, Ranganathan J. The Greenhouse Gas Protocol. The GHG Protocol for Project Accounting. World Business Council for Sustainable Development and World Resources Institute. 2005.
[Google Scholar]
- Lawrence PJ, Smith GR, Sullivan MJ, Mossman HL. Restored saltmarshes lack the topographic diversity found in natural habitat. Ecol Eng. 2018;115:58-66.
[Crossref] [Google Scholar]
- Mossman HL, Davy AJ, Grant A. Does managed coastal realignment create saltmarshes with ‘equivalent biological characteristics’ to natural reference sites?. J Appl Ecol. 2012;49(6):1446-1456.
[Crossref] [Google Scholar]
- Li S, Xie T, Pennings SC, Wang Y, Craft C, Hu M. A comparison of coastal habitat restoration projects in China and the United States. Sci Rep. 2019;9(1):1-0.
[Crossref] [Google Scholar]
- Liu Z, Fagherazzi S, Cui B. Success of coastal wetlands restoration is driven by sediment availability. Commun Earth Environ. 2021;2(1):1-9.
[Crossref] [Google Scholar]
- Archer AW. World's highest tides: Hypertidal coastal systems in North America, South America and Europe. Sediment Geol. 2013;284:1-25.
[Crossref] [Google Scholar]
- Thorn MF, Burt TN. Sediments and metal pollutants in a turbid tidal estuary. Can J Fish Aquat Sci. 1983;40(S1):s207-15.
[Crossref] [Google Scholar]
- MacDonald MA, de Ruyck C, Field RH, Bedford A, Bradbury RB. Benefits of coastal managed realignment for society: Evidence from ecosystem service assessments in two UK regions. Estuar Coast Shelf Sci. 2020;244:105609.
[Crossref] [Google Scholar]
- Macreadie PI, Anton A, Raven JA, Beaumont N, Connolly RM, Friess DA, et al. The future of Blue Carbon science. Nat Commun. 2019;10(1):1-3.
[Crossref]
- Manning AJ, Langston WJ, Jonas PJ. A review of sediment dynamics in the Severn Estuary: influence of flocculation. Mar Pollut Bull. 2010;61(1-3):37-51.
[Crossref] [Google Scholar]
- French JR. Numerical simulation of vertical marsh growth and adjustment to accelerated sea‐level rise, north Norfolk, UK. Earth Surf Process Landf. 1993;18(1):63-81.
[Cross Ref], [Google Scholar]
- Mantz PA, Wakeling HL. Aspects of sediment movement near to bridgwater bar, bristol channel. Proc Inst Civ Eng. 1982;73(1):1-23.
[Crossref] [Google Scholar]
- Darbyshire EJ, West JR. Turbulence and cohesive sediment transport in the Parrett estuary. Turbulence: Perspectives on Flow and Sediment Transport. Wiley, Chichester. 1993:215-47.
[Google Scholar]
- Mcleod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ. 2011;9(10):552-560.
[Crossref] [Google Scholar]
- Mcowen CJ, Weatherdon LV, Van Bochove JW, Sullivan E, Blyth S, Zockler C, et al. A global map of saltmarshes. Biodivers Data J. 2017: e11764.
[Crossref]
- Ouyang X, Lee SY. Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences. 2014;11(18):5057-5071.
[Crossref] [Google Scholar]
- Moreno-Mateos D, Power ME, Comín FA, Yockteng R. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 2012;10(1):e1001247.
[Crossref]
- Moritsch MM, Young M, Carnell P, Macreadie PI, Lovelock C, Nicholson E, et al. Estimating blue carbon sequestration under coastal management scenarios. Sci Total Environ. 2021;777:145962.
[Crossref] [Google Scholar]
- Murray NJ, Clemens RS, Phinn SR, Possingham HP, Fuller RA. Tracking the rapid loss of tidal wetlands in the Yellow Sea. Front Ecol Environ. 2014;12(5):267-72.
[Crossref] [Google Scholar]
- Mossman HL, Sullivan MJP, Dunk RM, Rae S, Sparkes RT, Pontee, N. Created coastal wetlands as carbon stores: potential challenges and opportunities In: Humphreys J Challenges in Estuarine andCoastal Science: Estuarine and Coastal Sciences Association 50th Anniversary Volume. UK: Pelagic Publishing. 2021.
- Needelman BA, Emmer IM, Emmett-Mattox S, Crooks S, Megonigal JP, Myers D, et al. The science and policy of the verified carbon standard methodology for tidal wetland and seagrass restoration. Estuaries Coast. 2018;41(8):2159-71.
[Crossref] [Google Scholar]
- Pontee NI. Impact of managed realignment design on estuarine water levels. P I Civil Eng-Mar En 2015; 168(2): 48-61.
[Crossref] [Google Scholar]
- Rowell DL. Soil science: Methods and applications. Routledge. 2014.
[Crossref] [Google Scholar]
- Sparkes RB, Lin IT, Hovius N, Galy A, Liu JT, Xu X, et al. Redistribution of multi-phase particulate organic carbon in a marine shelf and canyon system during an exceptional river flood: Effects of Typhoon Morakot on the Gaoping River–Canyon system. Mar Geol. 2015;363:191-201.
[Crossref] [Google Scholar]
- Team RC. R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. 2011.
- Pontee NI, Serato B. Nearfield erosion at the steart marshes (UK) managed realignment scheme following opening. Ocean Coast Manag. 2019;172:64-81.
[Crossref] [Google Scholar]
- Ranwell DS. Spartina salt marshes in southern England: II. Rate and seasonal pattern of sediment accretion. J Ecol. 1964:79-94.
[Crossref] [Google Scholar]
- Salzman J, Bennett G, Carroll N, Goldstein A, Jenkins M. The global status and trends of Payments for Ecosystem Services. Nat Sustain. 2018;1(3):136-144.
[Crossref] [Google Scholar]
- Saderne V, Geraldi NR, Macreadie PI, Maher DT, Middelburg JJ, Serrano O, et al. Role of carbonate burial in Blue Carbon budgets. Nat Commun. 2019;10(1):1-9.
[Crossref]
- Environment Agency. Steart Coastal Management Project Environmental Statement: Report produced by Halcrow for the Environment Agency. Bristol, UK. 2011:178
- Scott J, Pontee N, McGrath T, Cox R, Philips M. Delivering large habitat restoration schemes: lessons from the Steart Coastal Management Project. Coastal Management: Changing coast, changing climate, changing minds. 2016: 663-674. ICE Publishing.
[Crossref] [Google Scholar]
- Schuerch M, Spencer T, Temmerman S, Kirwan ML, Wolff C, Lincke D, et al. Future response of global coastal wetlands to sea-level rise. Nature. 2018;561:231-4.
[Crossref]
- Serrano O, Lovelock CE, B Atwood T, Macreadie PI, Canto R, Phinn S, et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat Commun. 2019;10(1):1-0.
[Crossref]
- Spearman J. The development of a tool for examining the morphological evolution of managed realignment sites. Cont Shelf Res. 2011;31(10):S199-S210.
[Crossref] [Google Scholar]
- Clapp J. Managed realignment in the Humber estuary: factors influencing sedimentation. University of Hull, Yorkshire, UK. 2009.
[Google Scholar]
- Spencer KL, Carr SJ, Diggens LM, Tempest JA, Morris MA, Harvey GL. The impact of pre-restoration land-use and disturbance on sediment structure, hydrology and the sediment geochemical environment in restored saltmarshes. Sci Total Environ. 2017;587:47-58.
[Crossref] [Google Scholar]
- Spencer T, Friess DA, Möller I, Brown SL, Garbutt RA, French JR. Surface elevation change in natural and re-created intertidal habitats, eastern England, UK, with particular reference to Freiston Shore. Wetl Ecol Manag. 2012;20(1):9-33.
[Crossref] [Google Scholar]
- Stewart-Sinclair PJ, Purandare J, Bayraktarov E, Waltham N, Reeves S, Statton J, et al. Blue restoration–building confidence and overcoming barriers. Front Mar Sci. 2020;7:748.
[Crossref] [Google Scholar]
- Sullivan MJ, Lewis SL, Affum-Baffoe K, Castilho C, Costa F, Sanchez AC, et al. Long-term thermal sensitivity of Earth’s tropical forests. Science. 2020;368(6493):869-874.
[Crossref]
- da Silva LV, Everard M, Shore RG. Ecosystem services assessment at Steart Peninsula, Somerset, UK. Ecosyst Serv. 2014;10:19-34.
[Crossref] [Google Scholar]
- R Core Team . Vienna: R Foundation for Statistical Computing. 2018.
- Hijmans RJ. Raster: geographic data analysis and modeling. R package. 2020:3-6.
- Wedding LM, Moritsch M, Verutes G, Arkema K, Hartge E, Reiblich J, et al. Incorporating blue carbon sequestration benefits into sub-national climate policies. Glob Environ Change. 2021;69:102206.
[Crossref] [Google scholar]
- Wollenberg JT, Ollerhead J, Chmura GL. Rapid carbon accumulation following managed realignment on the Bay of Fundy. Plos one. 2018;13(3):e0193930.
[Crossref] [Google scholar]
Author Info
Solomon I. Ubani*
Department of Nature Sciences, Gaiasce Company and Gss Subsidiary, United Kingdom
Citation: Ubani SI (2022) Fungal Mitigation of Sodium Chloride and Chloroform of Rivers and Canals. Fungal Genom Biol. 12:182.
Received: 22-Feb-2022, Manuscript No. FGB-22-15973;
Editor assigned: 25-Feb-2022, Pre QC No. FGB-22-15973 (PQ);
Reviewed: 11-Mar-2022, QC No. FGB-22-15973;
Revised: 18-Mar-2022, Manuscript No. FGB-22-15973 (R);
Published:
25-Mar-2022
, DOI: 10.35841/2165-8056.22.12.182
Copyright: © 2022 Ubani SI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.