Review Article - (2016) Volume 8, Issue 2
Gallic Acid: A Promising Lead Molecule for Drug Development
- Naira Nayeem1, Asdaq SMB2*, Heba Salem1 and Said AHEl-Alfqy1
- 1Department of Pharmaceutical Chemistry, College of Pharmacy, Northern Border University, Saudi Arabia
- 2College of Pharmacy, Al-Maarefa Colleges for Science and Technology, Riyadh, Saudi Arabia
*Corresponding Author:
Asdaq SMB, College of Pharmacy, Al-Maarefa Colleges for Science and Technology, Riyadh, Saudi Arabia, Tel: +91-80-65973260
Email:
Abstract
Gallic acid and its congeners are commonly present in variety of fruits and number of plants. In addition to its natural origin, large number of synthesized gallic acid derivatives are also available. It has a wide range of industrial uses including its role as standard for determing phenolic content of analytes in pharmaceutical industry, as source material for ink, paints and colour developer. Studies on gallic acid and its derivatives have exhibited its potential for combating oxidative damages, cancer manifestations and microbial infestations. Further, gallic acid extracted from different natural sources has been implicated to possess potency to ameliorate neurodegenerative disorders and aging. Furthermore, large number of reseach explorations are available to show its ability for the treatment of diabetes, ischemic heart diseases, ulcer and other ailments. In this review, an attempt is made to compile the scattered information on gallic acid and its derivatives for their pharmacological role, isolation and extraction procedures as well as quantification. This might help our research fraternity to explore gallic acid in their future research as a lead compound for new drug development.
<
Keywords:
Gallic acid; Isolation; Pharmacological activities; Quantification
Introduction
Medicinal plants are of great importance to health due to the presence of phytoconstituents. The most important of these constituents are alkaloids, glycosides, tannins, flavonoids, and phenolic compounds [1]. Phenolic acids are diverse group that includes hydroxybenzoic and hydroxycinnamic acids. Various phenolic acids reported from plants are ferulic acid, ellagic acid, synergic acid, caffeic acid etc. They are also of interest in food, cosmetic and pharmaceutical industries as well as substitutes for synthetic antioxidants [2]. One such prominent phenolic acid is gallic acid which is found in a wide variety of vegetables, fruits, tea, coffee and wine. It occurs in plants in the form of free acids, esters, catechin derivatives and hydrolysable tannins. It also occur as methylated gallic acids e.g., syringic acid or galloyl conjugates of catechin derivatives, i.e., flanvan-3-ols, or polygalloyl esters of glucose, quinic acid or glycerol [3,4]. Gallic acid has been reported to elicit various biological activities such as antibacterial, anti-fungal, antiviral, anti-inflammatory, antioxidant, anticancer, anti-diabetic etc.
Gallic acid (GA) is a phenolic compound. It is chemically known as 3, 4, 5-trihydroxybenzoic acid. The structure of gallic acid has phenolic groups that are a source of readily available hydrogen atoms so that radicals produced can be delocalized over the phenolic structure [5]. The interest in these compounds is due to its pharmacological activity as radical scavengers. It has been proved to have potential preventive and therapeutic effects in many diseases, where the oxidative stress has been implicated, including cardiovascular diseases, cancer, neurodegenerative disorders and in aging [6,7]. Due to these activities gallic acid could be considered as a promising lead compound for new drug development. Current work is an attempt to compile literature reporting on isolation, quantification and pharmacological activities of gallic acid and its derivatives to provide quick access to research scholars for their research exploration on gallic acid.
Occurrence
Gallic acid has been reported to occur in a number of plants, some of them are Allan blackia floribunda, Garcinia densivenia, Bridelia micrantha, Caesalpinia sappan, Dillenia indica, Diospyros cinnabarina, Paratecoma peroba, Psidium guajava, Syzygium cordatum, Rhus typhina, Tamarix nilotica, Vitis vinifera, Hamamelis virginiana, Toona sinensis Oenothera bienni and Rubus suavissimus [8].
Many gallic acid derivatives occur naturally in plant, these include 3-O-β-D-glucopyranoside (3-glucogallic acid) and 3-O-(6- galloylglucoside), 4-O-(6-galloylglucoside) from rhubarb, mudanoside B from Paeonia suffruticosa, 3-O-dodecanoyl (3-lauroylgallic acid) with antioxidant and antimicrobial activities from Satakentia liukiuensis, 3-methyl ether from Geranium collinum and Atraphaxis frutescens, 3-methyl-5-O-sulfate (as salts) from Frankenia laevis and Tamarix amplexicaulis, 3-methyl-4-O-[3,4-dihydroxy-5-methoxybenzoyl-(→6)- β-D-glucopyranoside] from Polygonum bistorta, 3-methyl-5-O-β-D-glucopyranoside from Tabernaemontana cymosa, 3-methyl ether from Poupartia axillaris and Rhus glabra, 3-ethyl ether from Phyllanthus emblica, and 4-ethyl ether from Mimosa hamata, Haematoxylum campechianum, Arbutus unedo, Eucalyptus gunnii, Terminalia chebula and Elephantorrhiza elephantina [8]. A recent study indicated presence of antioxidant gallic acid esters (gallates) in dust from homes and microenvironments [9].
Synthetic Derivatives
Gallic acid derivatives have been synthesized and reported to possess number of biological and pharmacological activities. The alkyl esters of gallic acid have been reported to possess anticancer, antioxidant ability and neuroprotective effect [10], scavenging free radicals [11], inducing apoptosis of cancer cells [12], inhibiting squalene epoxidase [13], interfering the signal pathways involving Ca2+ and oxygen free radicals [14]. The schiff bases of gallic acid were synthesized and reported for analgesic, anti-inflammatory and anticonvulsant activities [15]. Gallic acid-based indanone derivatives have been prepared and reported to possess anticancer activity [16]. A series of 2-(3,4,5-trihydroxy phenyl)-5-aryl-1,3,4-oxadiazole were synthesized and evaluated for their anti-tubercular activity [17]. A series of gallic hydrazones containing an indole moiety were evaluated for cytotoxic and antioxidant activities [18]. Derivatives such as 5-{6-(substituted phenyl)-5,6-dihydro-(1,2,4) triazolo(3,4-b)(1,3,4) thiadiazol-3-yl}benzene-1,2,3-triol were synthesized and screened for antimicrobial and anti-inflammatory effects [19]. A series of 33 gallic acid derivatives were synthesized and evaluated for antibacterial and antifungal activities [20]. Other derivatives of gallic acid such as 3,4-methylenedioxyphenyl 3,4,5-trihydroxybenzoate (GD-1) and S-(3,4-methylenedioxyphenyl)-3,4,5-trihydroxy-thiobenzoate (GD-3) were evaluated for cell death-inducing activity in cancer cell lines [21]. A series azo gallic acid complexes were prepared and evaluated for their antimicrobial activity [22].
Isolation
Gallic acid (GA) has been isolated from a number of plants. It has been extracted from rind, seed, stem, fruit, leaves, bark, wood etc. The solvents generally used are water, ethanol and methanol. Some of the plant extract from which GA has been isolated are methanolic extract of whole plant of Bergia suffruticosa, aqueous ethanolic extract of leaves of Ceratonia siliqua, methanolic extract of the leaves of Tectona grandis as well as methanolic extracts of bark, wood, leaf and fruits of Casuarina equisetifolia [23-25]. A bioassay guided isolation and identification of gallic acid derivatives epicatechin-(2 β → O → 7, 4 β → 8)-catechin (proanthocyanidin A1) and epicatechin-(β → 2 O → 7, 4 β → 8)-epicatechin (proanthocyanidin A2) were reported from peanut skin [26].
Structures of Gallic Acid and Some of its Derivatives
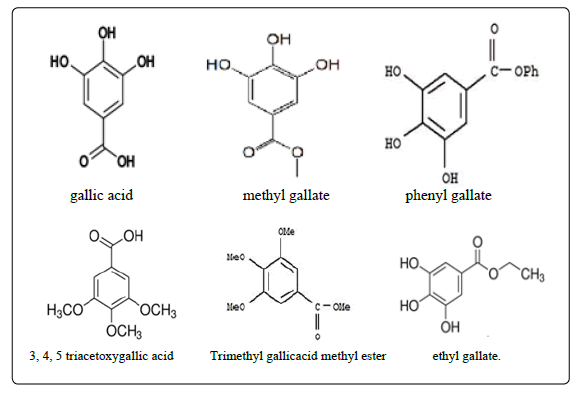
Quantification
Various methods have been reported for the quantification of GA in herbal raw materials and formulations which include TLC, HPLC, HPTLC, RP-HPLC etc. Gallic acid has been quantified from the flower buds of Syzygium aromaticum by TLC [27]. Further, HPLC technique is use for quantification of gallic acid in Ocimum sanctum [28]. Furthermore, UHPLC method was used for quantifying gallic acid in methanolic, ethanolic and hydroalcoholic extract of the seed of Cornus officinalis [29]. Gallic acid was quantified in hydroalcoholic extract of dried flowers of Nymphaea stellata and Eucalyptus hybrida by HPTLC [30]. Another study showed use of RP-HPLC method for quantification of GA in Symplocos racemose [31].
Pharmacology
There are several reports for the pharmacological activities of gallic acid and its derivatives. Review of literature reveals hepatoprotective potential of gallic acid in alleviating paracetamol-induced liver damage in mice, hepatic ischemia reperfusion injury in rats, CCl4-induced acute liver injury in rats, sodium fluoride-induced oxidative stress, acute liver damage induced by CCl4 [32,33], N nitroso-compounds-induced mutagenicity as well as obviating mouse lung adenomas by amines or ureas plus nitrite and by nitroso compounds [34]. GA has been reported to suppress cell viability, proliferation, invasion and angiogenesis in human glioma cells, inhibits the growth of HeLa cervical cancer cells via apoptosis and necrosis, induces apoptosis in tumoral cell lines and inhibit lymphocyte proliferation, inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells, causes inactivating phosphorylation via ATM-Chk2 activation, leading to cell cycle arrest [35-38]. It is also reported to possess anti-oxidant activity [39].
It has been reported that GA has anti-microbial activity against methicillin-resistant Staphylococcus aureus and Helicobacter pylori [40,41]. Anti-inflammatory actitivity has been evaluated by zymosaninduced acute food pad swelling in mice, carrageenan-induced paw edema, acetic acid-induced writhing responses and formalin-induced pain in animal models as reported in numerous publications and the suggested mechanisms were scavenging of superoxide anions, inhibition of myeloperoxidase release and activity as well as interference with activity of NADPH-oxidase [42-44]. The other reported activities are anti depressant [45], antiparkinson [46], anti diabetic [47], anti malarial [48], diuretic [49], cardioprotective [50], anti-viral [51], antifungal [52], wound healing [53], anthelmintic [54] and anxiolytic [55]. Gallic acid, when combine with other natural products such as, calycosin, reported to synergistically attenuate neutrophil infiltration and subsequent injury in isoproterenol-induced myocardial infarction [56].
Other Uses of Gallic Acid
As described above, gallic acid is routinely employed in pharmaceutical industry as standard for establishing phenolic content of analyte. One of the recent report indicated estimation of phenolic content in the extract of stinging nettle (Urtica dioica. L) was done in comparison with gallic acid equivalent/g [57]. Another research exploration on different drying treatments for green tea also evaluated total phenolic content based on gallic acid equivalent [58]. Further, Rosmarinic Acid, a New Polyphenol from Baccaurea ramiflora Lour. leaf was also subjected for estimating total phenolic content based on gallic acid equivalent [59].
Conclusion
To conclude, it is evident that gallic acid and its derivatives play a pivotal role in imparting medicinal properties of the plant and therefore it is considered as promising lead molecule for new drug development. Thus, it is imperative to promote more credible research on exploring medicinal properties of gallic acid and its congeners. Eventhough, in the the last few years there has been an increase in the number of publications on gallic acid, it might be more appropriate to carryout such research on human subjects following established system of standardization.
References
- Hill AF (1952) Economic Botany. A textbook of useful plants and plantproducts,(2nd edn.) McGarw-Hill Book Company Inc, NY.
- Soong YY, Barlow PJ (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food 88: 411-417.
- Tang HR, Covington AD, Hancock RA (2003) Structure-activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 70:403-413.
- Tang HR, Covington AD, Hancock RA (2003) Synthesis and spectroscopic characterisation of polygalloyl esters of polyols: models for gallotannins.JSoc Leather Chem Tech 87: 179-188.
- Nikolic KM (2006) Theoretical study of phenolic antioxidants properties in reaction with oxygen-centered radicals. J Mol Struc THEOCHEM 774:95-105.
- Karamaæ MA, Kosiñska, Pegg RB (2005) Comparison of radical-scavenging activities of selected phenolic acids.Pol J Food Nutr Sci 14:165-170.
- Kaur S, Michael H, Arora S, Harkonen PL, Kumar S (2005) The in vitro cytotoxic and apoptotic activity of Triphala -an Indian herbal drug. J Ethnopharm 97: 15-20.
- Shahriar K, Robin JM (2010) Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 15: 7985-8005.
- Wang W, Asimakopoulos AG, Abualnaja KO, Covaci A, Gevao B, et al. (2016) Synthetic phenolic antioxidants and their metabolites in indoor dust from homes and microenvironments. Environ Sci Technol50: 428-34
- Locatelli C, Filippin MFB, Creczynski PTB (2013) Alkyl esters of gallic acid as anticancer agents: a review. Eur J Med Chem 60:233-239.
- Dwibedy P, Dey GR, Naik DB, Kishore K, Moorthy PN (1999) Pulseradiolysis studies on redox reaction of gallic acid: one electron oxidation of gallic acid by hallic acid OH adduct. Phys Chem Chem Phys 1: 1915-1918.
- Saeki K, You A, Isemura M, Abe I, Seki T, et al. (2000) Apoptosis inducing activity of lipid derivatives of gallic acid. Biol Pharm Bull 23: 1391-1394.
- Abe I, Seki T, Noguchi H (2000) Potent and selective inhibition of squalene epoxidase by synthetic gallic esters. Biochem Biophys Res Commun 270: 137-140.
- Sakaguchi N, Inoue M, Ogihara Y (1998) Reactive oxygen species and intracellular Ca2+, common signals for apoptosis induced by gallic acid.Biochem Pharmacol 55: 1973-1981.
- Kumara Prasad SA, Subrahmanyam EVS, Shabaraya A (2014) Design and biological screening of some novel formazan derivatives from schiff bases of gallic acid.World Journal of Pharmaceutical Research 3: 2741-2752.
- Saxena HO, Faridi U, Srivastava S, Kumar JK, Darokar MP, et al. (2008) Gallic acid-based indanone derivatives as anticancer agents.Bioorg Med Chem Lett 18: 3914-3918.
- Arunkumar S, Ilango K, Manikandan RS, Sudha M, Ramalakshmi N (2009) Synthesis, Characterisation and BiologicalEvaluation of some novel 2,5-Disubstituted-1,3,4-oxadiazole derivatives of Gallic acid.International Journal of ChemTech 1: 1094-1099.
- Khaledi H, Alhadi AA, Yehye WA, Ali HM, Abdulla MA, et al. (2011) Antioxidant, Cytotoxic Activities, and Structure-activity relationship of gallic acid-based indole Derivatives.Arch Pharm (Weinheim) 344: 703-709.
- Arunkumar S, Ilango K, Ravindar B, Ramalakshmi N (2009) Synthesis and biological evaluation of some novel triazolo, thiadiazole derivatives of gallic acid. Der Pharma Chemica 1:70-77.
- Anurag K, Arun N, Pradeep K, Balasubramanian N (2013) Synthesis, antimicrobial evaluation and QSAR studies of gallic acid derivatives. Arabian Journal of Chemistry.
- Sakaguchi N, Inoue M, Isuzugawa K, Ogihara Y, Hosaka K (1999) Cell Death-Inducing Activity by Gallic Acid Derivatives.BiolPharm Bull22: 471-475.
- Mamdouh SM, Sawsan SH, Alaa EA, Nessma MN (2012) Synthesis and spectroscopic characterization of gallic acid and some of its azo complexes.Journal of Molecular Structure 1014: 17-25.
- Sheetal A, Honnegowda S, Mandapati R (2007) Isolation and TLC Densitometric Quantification of Gallicin, Gallic Acid, Lupeol and ß-Sitosterol from Bergia suffruticosa, a Hitherto Unexplored Plant. Chromatographia 66:725-734.
- Naira Nayeem, Karvekar MD (2010) Isolation of phenolic compounds from the methanolic extract of Tectona grandis.Res J Pharma Bio Chem Sci1: 221.
- Aher AN, Pal SC,Yadav SK, Patil UK, Bhattacharya S (2010) Isolation and Characterization of Phytoconstituents from Casuarina equisetifolia (Casuarinaceae). Asian J Chem 22: 3429-3434.
- Oldoni TL, Melo PS, Massarioli AP, Moreno IA, Bezerra RM, et al. (2016) Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem 192:306-312.
- Pathak SB, Niranjan K, Padh H, Rajani M (2004) TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove.Chromatographia60: 241-244.
- Shafqatullah, Khan R, Hassan W, Hussain A, Asadullah, et al. (2014) Development of HPLC method by UV-VIS detection for the quantification of phenolic acids in different Ocimum sanctum Linn. extracts.Pak J Pharm Sci 27: 1271-1275.
- Liang LZ, Yong MW, Man X, Dong MW, Jia HC (2014) Quantification of gallic acid and ellagic acid from the seed of Cornus Officinalis by UHPLC METHOD and their antioxidant activity.Chem Eng Comm201:545-556.
- Sachin UR, Salunkhe VR, Dhabale PN, Burade KB (2009) HPTLC Method for Quantitative Determination of Gallic Acid in Hydroalcoholic Extract of Dried Flowers of Nymphaea Stellata Willd.Asian J Research Chem 2.
- Nagore DH, Patil P,Sagulale AD, Deshmukh TA (2012)Validated RP-HPLC Method for Quantification of Gallic Acid as marker in Different Extracts of Symplocos racemosa (Roxb) International Journal of Chromatographic Science 2(4): 19-23.
- Kanai S, Okano H (1998) Mechanism of the protective effects of sumac gall extract and gallic acid on the progression of CCl4-induced acute liver injury in rats.Am J Chin Med 26: 333-341.
- Seyed FN, Seyed MN, Solomon H, Akbar HM, Antoni S, et al. (2013) Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress.Industrial Crops and Products 44:50-55.
- Gichner T, Pospisil F, Veleminsky J, Volkeova V, Volke, L (1987) Two types of antimutagenic effects of gallic acid and tannic acids towards N nitroso- compounds-induced mutagenecity in the Ames Salmonella assay. Folia Microbiol 32: 55-62.
- Alyssa GS, Jeffrey HW, Hannah E, Esther FR, Ayelet RB, et al. (2013) Cytotoxic and proapoptotic activities of gallic acid to human oral cancer HSC-2 cells. Oxid Antioxid Med Sci 2: 265-274.
- Madlener S, Illmer C, Horvath Z, Saiko P, Losert A, et al. (2007) Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells.Cancer Lett 245: 156-162.
- Agarwal C, Tyagi A, Agarwal R (2006) Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther 5: 3294-3302.
- Wang K, Zhu X, Zhang K, Zhu L, Zhou F (2014) Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells.J Biochem Mol Toxicol 28: 387-393.
- Franziska F, Asima C, Tatjana S, Michael K, Siegfried K (2007)Antioxidant and free radical scavenging activities of sumac (Rhus coriaria) and identification of gallic acid as its active principle. BMC Pharmacology 7:A71.
- Borges A, Ferreira C, Saavedra MJ, Simões M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria.Microb Drug Resist 19: 256-265.
- Roberto DG, Remigio LS, Elias OS,Hector TA (2013) Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. Food Science and Technology 54: 331-335.
- Kroes BH, van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP (1992) Anti-inflammatory activity of gallic acid.Planta Med 58: 499-504.
- Angelica GC, Candida AL, KassuyaII, Joao BC, Petrovick PR (2013) Anti-inflammatory, antiallodynic effects and quantitative analysis of gallic acid in spray dried powders from Phyllanthus niruri leaves, stems, roots and whole plant.Rev bras farmacognosia 23: 124-131.
- Guan HH, Ming-HH, Chuan SC, Shyh-SH,Pei-HSH, et al. (2012) Analgesic and Anti-Inflammatory Activities of Aqueous Extracts of Fructus Ligustri Lucidi.Journal of Food & Drug Analysis20: 617-627.
- Chhillar R, Dhingra D (2013) Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress.Fundam Clin Pharmacol 27: 409-418.
- Prasad CN, Anjana T, Banerji A, Gopalakrishnapillai A (2010) Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells.FEBS Lett 584: 531-536.
- Griffith R, Chanphen R, Leach SP, Keller PA (2002) New anti-malarial compounds from database searching.Bioorg Med Chem Lett 12: 539-542.
- Ramya K, Mohandas SR, Ashok KJ (2014) Evaluation of diuretic activity of gallic acid in normal rats. Journal of Scientific and Innovative Research. 3: 217-220.
- Patel SS, Goyal RK (2011) Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats.Pharmacognosy Res 3: 239-245.
- Kratz JM, Andrighetti-Frahner CR, Kolling DJ, Leal PC, Cirne-Santos CC, et al. (2008) Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate.Mem Inst Oswaldo Cruz 103: 437-442.
- de Paula E Silva AC, Costa-Orlandi CB, Gullo FP, Sangalli-Leite F, de Oliveira HC, et al. (2014) Antifungal Activity of Decyl Gallate against Several Species of Pathogenic Fungi.Evidence-Based Complementary and Alternative Medicine: 506273.
- Nayeem N, Karvekar MD (2011) Stability studies and evaluation of the semi-solid dosage form of the rutin, quercitin, ellagic acid, gallic acid and sitosterol isolated from the leaves of Tectona grandis for wound healing activity. Archives of Applied Science Research3: 43.
- Ndjonka D, Abladam ED, Djafsia B, Ajonina-Ekoti I, Achukwi MD, et al. (2014 ) Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans.Helminthol88:481-488.
- Dhingra D, Chhillar R, Gupta A (2012) Antianxiety-like activity of gallic acid in unstressed and stressed mice: possible involvement of nitriergic system.Neurochem Res 37: 487-494.
- Cheng Y, Zhao J, Tse HF, Chris Le X, Rong J (2015)Plant Natural Products Calycosin and Gallic Acid Synergistically Attenuate Neutrophil Infiltration and Subsequent Injury in Isoproterenol-Induced Myocardial Infarction: A Possible Role for Leukotriene B4 12-Hydroxydehydrogenase?Oxidative Medicine and Cellular Longevity 434052.
- Adhikari BM, Bajracharya A, Shrestha AK (2015) Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours.Food Sci Nutr 4: 119-124.
- Roshanak S, Rahimmalek M, Goli SA (2016) Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J Food Sci Technol 53:721-729.
- Usha T, Middha SK, Bhattacharya M, Lokesh P, Goyal AK (2014) Rosmarinic Acid, a New Polyphenol from Baccaurea ramiflora Lour. Leaf: A Probable Compound for Its Anti-Inflammatory Activity.Antioxidants (Basel) 3: 830-842.
Citation: Nayeem N, Asdaq SMB, Salem H, AHEl-Alfqy S (2016) Gallic Acid: A Promising Lead Molecule for Drug Development. J App Pharm 8:213.
Copyright: © 2016 Nayeem N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.