
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2023)Volume 13, Issue 1
Intoxication as well as infectious and metabolic diseases resulting in liver and kidney damage is the major causes of losses in poultry species. While there are no specific drugs for reversal of pathophysiology of these organs, certain herbs such as garlic have been shown to improve livability of livestock and poultry. Its probable potential at enhancing the functionality of these organs in the face of injuries/insults by intoxication was investigated. Harco cockerels subgroup supplemented with 0.25% garlic-meal and administered a single dose of 300 mg/kg acetaminophen orally at 8 week-old (G1; n=25) and subgroup without acetaminophen (G2; n=25), those not supplemented and without acetaminophen (NG2; n=25) and those not initially supplemented but administered acetaminophen and later supplemented (NG1g; n=20) had higher Newcastle disease vaccinal antibody titers (8.0 ± 0.25, 7.5 ± 0.24, 7.6 ± 0.23, 8.14 ± 0.31, respectively) in comparison with subgroup without supplementation and administered acetaminophen (NGI; n=25, 7.13 ± 0.38). Serum protein levels had a similar pattern. AST levels (U/L) were significantly higher (p<0.05) in acetaminophen subgroups G1 (79.4 ± 5.79) and NG1 (83.7 ± 7.5) than their respective controls G2 (75.1 ± 7.85) and NG2 (65.2 ± 6.84) with no corresponding increase in CK levels. At 2, 7 and 14 days post-administration of acetaminophen (PAA), creatinine (mg/dl) was significantly higher in G1 (1.44 ± 0.01, 1.42 ± 0.01, 1.44 ± 0.02) and NG1 (1.47 ± 0.01, 1.51 ± 0.01, 1.47 ± 0.01) than in G2 (1.16 ± 0.05, 1.23 ± 0.01, 1.26 ± 0.06) and NG2 (1.31 ± 0.06, 1.29 ± 0.02, 1.31 ± 0.05). Similar pattern was observed at 21 days PAA. Clinicopathological observations associated with toxic dose of acetaminophen in liver and kidneys were reversed (NG1g). Thus, exhibiting the potential of garlic in protection against hepatorenal damage or injury.
Acetaminophen; Clinicopathology; Hepatoprotective; Hepatotoxicity; Nephroprotective; Nephrotoxicity
Liver and kidney are critical organs to the physiology and metabolism of the animal body. The liver has been described as a sophisticated chemical laboratory where numerous chemical transformations in the body take place. It produces some chemicals, modifies others to allow the body to use them and neutralizes an array of toxins. The avian liver is necessary for metabolism of carbohydrate and metabolites, drugs and chemicals, protein synthesis and has antimicrobial activities via the Kupffer cells, while the kidney is responsible for excretion of metabolites and osmoregulation [1]. However, certain conditions of poultry affect the normal functions of these organs with deleterious effects on the health and productivity of affected birds. Mycotoxicoses, fatty liver syndrome and certain infectious diseases such as fowl typhoid, pullorum disease, histomoniasis and marek’s disease affect the functioning of the liver and kidneys of poultry species [2,3].
While there are no specific drugs for the reversal of pathophysiology of the liver and kidney, certain herbs have been shown to improve the liveability of livestock and poultry in the practice of herbal medicine [4]. Some commonly used herbs include ginger, aloe vera, moringa, garlic and more. Plant extracts and spices can play a role in supporting both performance and health status of animals [5,6]. Beneficial effects of herbal extracts or active substances in animal nutrition may include the stimulation of appetite and feed intake, the improvement of endogenous digestive enzyme secretion, activation of immune response and antibacterial, antiviral, antioxidant, and anthelminthic actions [7]. Recent research works on herbal formulations as feed additives have shown encouraging results as regards weight gain, feed efficiency, lowered mortality, and increased liveability in poultry birds [8-11].
Garlic (Allium sativum) is both an herbal medicine and a spice belonging to the onion family Alliceae. It is used for the prevention and treatment of a variety of diseases [12]. Garlic has several beneficial effects for humans and animals, exhibiting antimicrobial, antioxidant, and anti-hypertensive, anti-inflammatory, and hypolipidaemic properties [13,14]. Ghazanfari T, et al. [15], observed significant increase in delayed type hyper-sensitivity response, but not in antibody response to sheep red blood cells in mice placed on garlic. However, Ismail IE, et al. [14], reported higher values of immunoglobulin M (IgM) and IgG while Elmowaldi GA, et al. [16], reported enhanced chicks’ innate immunity post-dietary supplementation with garlic.
In view of the benefits of garlic preparations in humans and animals alike, it’s probable potential at enhancing the functionality of the liver and kidney in poultry species, in the face of constant injuries or insults by any of the aforementioned factors is yet to be investigated. This study was therefore carried out to investigate the probable protective effects of garlic against liver and kidney damage or injuries in chickens.
Rearing of experimental chickens
One-day-old Harco cockerels (135) were purchased from a commercial hatchery in Ibadan, Nigeria out of which 15 were bled for the determination of Newcastle disease antibody titer. Rearing was carried out in open-sided tropical cages at the Department of Veterinary Medicine, University of Ibadan. The chicks were randomly divided into two groups of 50 (Garlic-G) and 70 (No Garlic-NG) chicks. Chicks in Group G were fed ration containing 0.25% patented garlic-meal NG/P/2012/285 from day-old, while those in Group NG were fed basal diet and served as the control group [17]. The garlic-meal comprised 70% raw garlic powder. The chicks were administered multivitamins in water for the first seven days of life. Infectious bursal disease vaccine was administered orally at 8 and 17 days of age, Newcastle disease vaccine, LaSota strain orally at 14 days of age while Newcastle disease vaccine, Komarov strain was administered intramuscularly at 9 weeks of age. Feed and water were always made available.
Induction of hepatotoxicity and nephrotoxicity
At 8 weeks of age, chickens in Group G were randomly divided into two sub-groups i.e., G1 and G2 of 25 each while those in group NG were also divided into two sub-groups NG1 and NG2 of 45 and 25 chickens, respectively. A single known toxic dose of acetaminophen (PANADOLR, Glaxosmithkline Consumer Nigeria Plc.), i.e., 300 mg/kg [18,19], was administered orally to each chicken in sub-groups G1 and NG1 after the determination of their average weight as 0.4 kg i.e., 120 mg in 1 ml distilled water per bird. At 9 weeks of age, 20 chickens were isolated from NG1, designated as NG1g and garlic meal was included in their feed at 0.25%.
Determination of Newcastle disease antibody titer
Twelve experimental chickens per group were bled (2 ml each) at 4, 8 (prior to administration of Acetaminophen), 9, 11 and 14 weeks of age via jugular venipuncture using 21 gauge needles and 5 ml syringes. This is in addition to blood samples collected at dayold. Blood samples were dispensed into plain sample bottles and allowed to clot at room temperature. Serum samples were harvested into 1.5 ml eppendorf tubes and Haemagglutination Inhibition (HI) test was conducted as described by OIE [20]. HI titers were read visually as the last well showing haemagglutination inhibition.
Serum enzymes and proteins assay
Twelve chickens per group were bled at 8 weeks of age, prior to administration of acetaminophen, as well as, at 2, 7, 14 and 21 days post administration. Sera were harvested and levels of aspartate aminotransferase, creatine kinase, creatinine, total protein, and albumin from the different sub-groups were determined by spectrophotometry using FORTRESS DIAGNOSTICSR kits (Fortress Diagnostics Limited, Antrim, BT41 1QS, UK) and following manufacturer’s manual. Globulin levels in serum samples were derived by deducting the values of albumin levels from those of total protein.
Clinical and pathological assessment
After the administration of acetaminophen, the chickens were monitored daily for clinical signs and mortality which was scored based on the degree of severity i.e., 1 for mild, 2 for moderate and 3 for marked [21]. Also, at 5, 8 and 14 days after the administration of acetaminophen, two chickens per group were euthanized by cervical dislocation [22]. Gross pathological changes observed were noted, tissue samples from liver and kidney were also harvested into 10% formalin and sectioned for histopathological examination.
Statistical analysis
Mean and Standard Error of Mean (SEM) for each parameter assessed was calculated per sub-group and analysed for significant differences between sub-groups using analysis of variance and least significance difference method of multiple comparison at p<0.05.
Newcastle disease vaccinal immune response
At day-old, the chicks had a maternal antibody Mean Geometric Titer (MGT) of 5.42 ± 0.2 (log2) which decreased to 3.1 ± 0.39 and 3.3 ± 0.23 in the No-Garlic (NG) and Garlic (G) groups, respectively, at 4 weeks of age (Figure 1). By 8 weeks of age MGT was 3.2 in all groups and decreased to a range of 2.1 ± 0.16 to 2.3 ± 0.25 by 9 weeks of age i.e., one-week post-administration of acetaminophen, with the G1 and G2 sub-groups having the highest titers with no significant difference (p>0.05) between them. By 11 weeks of age i.e., 3 weeks post-administration of acetaminophen, MGTs had increased to 7.13 ± 0.38, 8.14 ± 0.31, 7.6 ± 0.23, 8.0 ± 0.25 and 7.5 ± 0.24 in sub-groups NG1, NG1g, NG2, G1 and G2, respectively, while they were decreased to 3.75 ± 0.28, 4.0 ± 0.3, 5.4 ± 0.31, 6.3 ± 0.23 and 7.1 ± 0.18, respectively at 14 weeks of age.
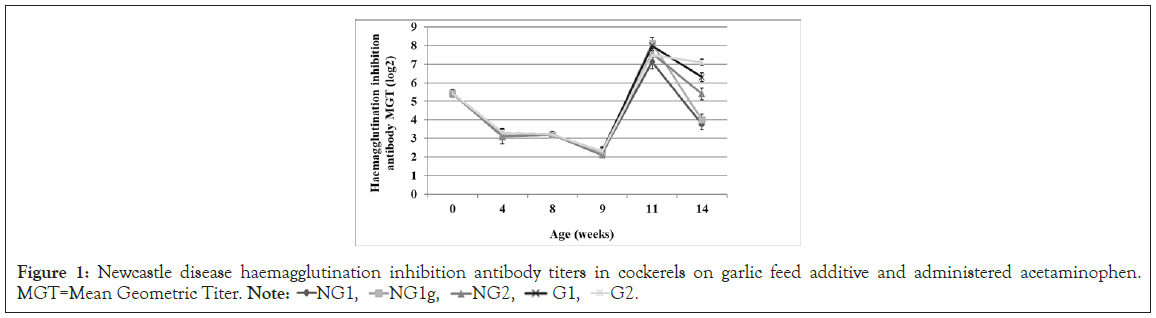
Figure 1: Newcastle disease haemagglutination inhibition antibody titers in cockerels on garlic feed additive and administered acetaminophen.
MGT=Mean Geometric Titer. Note:  NG1,
NG1,  NG1g,
NG1g,  NG2,
NG2,  G1,
G1,  G2.
G2.
Serum enzyme assay
The mean level of Aspartate Aminotransferase (AST) in the serum of no-garlic group NG was 62.4 ± 1.21 while it was 52.4 ± 1.1 in the garlic group G pre-administration of acetaminophen (Figure 2), with the difference being significant (p<0.05). At 2 days post-administration of acetaminophen (PAA), mean levels were 83.7 ± 7.5 and 62.5 ± 6.84 in sub-groups NG1 and NG2 (p<0.05), respectively, while they were 79.4 ± 5.79 and 75.1 ± 7.85 in sub-groups G1 and G2, respectively. At 7, 14 and 21 days PAA, AST levels ranged between 25.77 ± 2.9 (NG1) and 34.9 ± 2.74 (G2), 39.6 ± 4.79 (NG1g) and 57.2 ± 2.96 (NG1), as well as between 36.57 ± 5.02 (NG1g) and 65.3 ± 2.35 (NG1), respectively. The mean level of Creatine Kinase (CK) in the serum of no-garlic group NG was 548.2 ± 48.18 while it was 590.2 ± 40.82 in the garlic group G pre-administration of acetaminophen (Figure 2).
Figure 2: Serum aspartate aminotransferase, creatine kinase, creatinine, and albumin concentrations in cockerels pre and post-administration of toxic
dose of acetaminophen. Note:  NG1,
NG1, NG2,
NG2, G1,
G1,  G2,
G2,  NG1g.
NG1g.
At 2 days PAA, mean levels were 699.2 ± 52.3 and 700.2 ± 28.9 in sub-groups NG1 and NG2 respectively, while they were 712.8 ± 22.02 and 738.4 ± 41.6 in sub-groups G1 and G2, respectively. At 7 and 14 days PAA, CK levels ranged between 765.0 ± 10.7 (G1) and 532.0 ± 34.31 (NG1) and 401.0 ± 29.0 (NG1) and 547.0 ± 12.12 (G2), respectively.
The mean level of creatinine in the serum of no-garlic group NG was 0.42 ± 0.02 while it was 0.39 ± 0.03 in the garlic group G pre-administration of acetaminophen (Figure 2). At 2 days PAA, mean levels were 1.47 ± 0.01 and 1.31 ± 0.06 in sub-groups NG1 and NG2 respectively, while they were 1.44 ± 0.01 and 1.16 ± 0.05 in sub-groups G1 and G2, respectively. At 7, 14 and 21-days PAA, creatinine levels ranged between 1.23 ± 0.01 (G2) and 1.51 ± 0.01 (NG1), 1.26 ± 0.06 (G2) and 1.47 ± 0.01 (NG1) and 1.44 ± 0.13 (NG2) and 2.32 ± 0.07 (NG1), respectively.
Serum proteins assay
Pre-administration of acetaminophen, mean total protein concentration in the garlic group G was 3.7 ± 0.23 g/100 ml, while it was 3.75 ± 0.27 g/100 ml in the no-garlic group NG. At 2 days PAA, mean values were 5.34 ± 0.51 and 5.83 ± 0.51g/100 ml in subgroups NG1 and NG2 respectively, while they were 7.11 ± 0.31 and 7.05 ± 0.3 g/100 ml in sub-groups G1 and G2, respectively. At 7, 14 and 21 days PAA, total protein concentrations ranged between 3.09 ± 0.15 (NG1) and 3.79 ± 0.32 (NG2), 4.12 ± 0.34 (NG2) and 5.64 ± 0.42 (G2) and 5.2 ± 0.34 (NG1g) and 6.45 ± 0.22 g/100 ml (G2), respectively.
Pre-administration of acetaminophen mean serum albumin concentration in the garlic group G was 0.81 ± 0.05 g/100 ml, while it was 0.51 ± 0.09 g/100 ml in the No-Garlic group NG (Figure 2). At 2 days PAA, mean values were 1.46 ± 0.08 and 1.58 ± 0.17 g/100 ml in sub-groups NG1 and NG2 respectively, while they were 1.55 ± 0.08 and 1.81 ± 0.08 g/100 ml in sub-groups G1 and G2, respectively. At 7, 14 and 21 days PAA, mean albumin concentrations ranged between 0.91 ± 0.09 (NG1) and 1.13 ± 0.02 (G2), 0.51 ± 0.12 (NG1g) and 0.81 ± 0.07 (G2) and 1.15 ± 0.03 (NG1) and 1.28 ± 0.07 g/100 ml (G2), respectively.
Pre-administration of acetaminophen mean globulin concentration in the garlic group G was 2.89 ± 0.24 g/100 ml, while it was 3.24 ± 0.28 g/100 ml in the no-garlic group NG (Figure 3). At 2 days PAA, mean values were 3.76 ± 0.44 and 4.36 ± 0.54 g/100 ml in groups NG1 and NG2 respectively, while they were 5.56 ± 0.3 and 5.25 ± 0.31 g/100 ml in groups G1 and G2, respectively. At 7, 14 and 21 days PAA, mean globulin concentrations ranged between 2.07 ± 0.14 (NG1) and 2.74 ± 0.29 (NG2), 3.45 ± 0.41 (NG2) and 5.04 ± 0.31 (G1) and 3.77 ± 0.24 (NG1) and 5.12 ± 0.45 (G1) g/100 ml, respectively.
Figure 3: Serum globulin concentrations in cockerels pre- and post-administration of toxic dose of acetaminophen. Note:  NG1,
NG1,  NG2,
NG2,  G1,
G1, G2,
G2,  NG1g.
NG1g.
Clinical assessment
Clinical signs observed were anorexia, dullness, paleness of combs and wattles, whitish diarrhoea and jaundice. These were visible from day 4 PAA. Total scores of these clinical signs are presented in Figure 4 as 7, 11 and 12 for NG1; 0, 1 and 1 for NG2; 0, 2, 0 for G1 and 0, 0, 0 for G2 at 5, 8 and 14 days PAA, respectively, while NG1g had a total score of 8 at 14 days PAA. Scores for anorexia in NG1 at 5, 8 and 14 days PAA were 2, 3 and 3 respectively; while G1 had a score of 1 at 8-days PAA and NG1g had a score of 2 at 14 days PAA. Anorexia was not observed in NG2 and G2 throughout the period of the experiment. Paleness was scored as 2, 3 and 2 at 5, 8 and 14 days PAA, respectively, in Group NG1, 1 at both 8 and 14 days PAA in NG2, and 1 at 8-days PAA in G1. Also, dullness was scored as 1, 3 and 3 in NG1 at 5, 8 and 14 days PAA, respectively, while it was 2 at 14 days PAA in NG1g. Chickens in groups NG2, G1 and G2 did not exhibit dullness throughout the period of the experiment. NG1 exhibited watery-white feaces with a score of 2 at 14 days PAA while yellowish discolouration of combs, beak and wattles, with a score of 2 was observed in one chicken at 5, 8 and 14 days PAA. Two mortalities were recorded in NG1g by day-14 PAA.
Figure 4: Total score of clinical signs and gross pathology observed in cockerels on garlic meal feed-inclusion post-administration of acetaminophen. Note:  5 days PAA,
5 days PAA,  8 days PAA,
8 days PAA,  14days PAA.
14days PAA.
Gross pathology
The carcasses of the two mortalities recorded were emaciated. They had swollen and congested liver, markedly engorged gall bladder and slightly swollen kidneys. From the euthanized cockerels, gross lesions observed were haemorrhages in skeletal muscle and hock joint, swollen and congested liver, engorged gall bladder and swollen kidneys (Figure 5). Total score for gross pathology was 10, 8, 8 for NG1; 1, 0, 2 for NG2; 6, 3, 2 for G1 and 0, 0, 0 for G2 at 5, 8 and 14 days PAA, respectively, while NG1g had a total score of 6 at 14 days PAA (Figure 4). Moderate (2) and mild (1) haemorrhages were observed in NG1 and G1, respectively, at 5 days PAA. In NG1, swollen and congested liver had scores of 3, 3 and 2 at 5, 8 and 14 days PAA, NG2 had a score of 1 at 14 days PAA while G1 had a score of 1 in each of 5, 8 and 14 days PAA. Engorged gall bladder was marked (3) at 5, 8 and 14 days PAA in NG1 and mild (1) at 5 and 14 days PAA in NG2 while it was marked (3) at 5 days PAA and mild (1) at both 8 and 14 days PAA in G1. NG1 had scores of 2, 2 and 3 for swollen kidneys at 5, 8 and 14 days PAA, respectively, while G1 had a score of 1 at both 5 and 8 days PAA.
Figure 5: Gross pathological lesions observed in cockerels fed garlic-meal and administered acetaminophen. Note: A: Ecchymotic haemorrhage (arrows) in the inguinal region (A1) and hock joint (A2) of 8 week-old cockerels at 5 days post-administration of acetaminophen (NG1). B1: Engorged gall bladder (arrow) extended beyond the liver margin in 8 week-old cockerel on garlic feed-inclusion and administered acetaminophen (G1). B2: Liver and gall bladder from 9 week-old cockerels. NG1 showing remarkably more engorged gall bladder (arrow) than G1 (both groups at 8 days post-administration of acetaminophen). C: Left carcass-NG2. Right carcass-NG1, showing congested and swollen liver (arrow).
Histopathology
Vacuolar degeneration and necrosis of hepatocytes in NG1 and coagulative necrosis of the hepatocytes mainly in G1, as well as infiltration of mononuclear cells (largely plasma cells) were observed at 5 and 14 days PAA (Figure 6). Also, kidney tissues showed necrosis of tubular epithelial cells, glomerular degeneration and infiltration of mononuclear cell consisting mainly of plasma cells (Figure 7). Necrosis and cellular degeneration were more severe in NG1 than in G1 while cellular infiltration was more abundant in G1 than in NG1. On the contrary, no such lesions were observed in tissues from NG2 and G2. Liver and kidney sections obtained from chickens that were separated from NG1 and administered garlic i.e., NG1g, showed milder cellular necrosis and fewer mononuclear cell infiltration at 14 days PAA i.e., 6 days post-inclusion of garlic in feed (Figure 8).
Figure 6: Photomicrograph of liver (X400) of 8 week-old cockerels, 5 days post-administration of toxic dose of acetaminophen (300 mg/kg body weight- NG1 and G1) showing vacuolar degeneration (a), coagulative necrosis (b), and mononuclear cells infiltration (c).
Figure 7: Photomicrograph of kidneys of 8 week-old cockerels, 5 days post-administration of toxic dose of acetaminophen (300 mg/kg body weight-NG1 and G1) showing tubular epithelial necrosis (a), glomerullar degeneration (b), and mononuclear cells infiltration (c).
Figure 8: Photomicrograph of the liver (top) and kidney (bottom) of 10 week-old cockerels, 14 days post-administration of toxic dose of acetaminophen (300 mg/kg body weight) and 6 days post-feeding with garlic-meal (NG1g), showing milder cellular necrosis and less mononuclear cells infiltration in NG1g (X400).
This study investigated the probable protective effect of garlic against liver and kidney damage/injury in chickens. Conditions that affect the normal functioning of these two organs will have deleterious effect on health and productivity of poultry.
Newcastle disease vaccinal immune response
Apart from Kupffer cells and T cells in the liver as immune cells, there are IgM bearing B cells which produce immunoglobulins against ingested antigens that will eventually pass through the liver via the portal circulation [23]. Since these experimental chickens were vaccinated against Newcastle disease via the oral route, assessment of vaccinal response as an indicator of liver function is therefore pertinent. Newcastle disease antibody assay showed that by 3 weeks PAA (11 week-old), the No-Garlic/acetaminophen group (NG1) had the least MGT of 7.13 ± 0.38 which was significantly lower (p<0.05) than those of the other groups, while NG1g which is the sub-group of NG1 that was placed on garlic-meal from 1 week PAA, had the highest MGT of 8.14 ± 0.31. Also, by 6 weeks PAA (14 week-old), the garlic groups with and without administration of acetaminophen had the highest MGTs. Although Elbaz AM, et al. [24], did not record significant influence of garlic on ND antibody response, the results of the present study has again highlighted the immunomodulatory effect of garlic as earlier reported by Oladele OA, et al. [21], Arreola R, et al. [25], and Percival SS, [26]. Percival SS, [26], reported that garlic activates NK cells and improves the function of T-cells. Also, although Jafari RA, et al. [27], reported that garlic supplementation in feed of chicks did not influence antibody response to ND vaccination which is contrary to the findings in this study, it should be noted that the routes of vaccine administration are different. While Jafari RA, et al. [27], used the intraoccular route, the oral route was used in this study. Antibody response to ingested antigen is dependent on the functionality of the liver due to its supply by the portal circulation. The liver is considered an organ of immunology as such, any injury to it will reduce the immunocompetence of an animal, hence the decreased ND antibody response recorded in chickens in group NG1 [28]. Inspite of the induction of hepatotoxicity by acetaminophen, chickens in group G1 were able to mount a significantly higher level (p<0.05) of ND antibody response than the NG1 group, indicating the probable potential of garlic at preserving the immune function of the liver in the event of a hepatotoxic condition. Also, the higher level of antibody response in the NG1g group which was placed on garlic-meal after the induction of hepatotoxicity shows that garlic has the potential to enhance regeneration of liver tissue after an injury as suggested by Memudu AE, et al. [29], with regards to testicullar cells.
Serum enzymes
Pre-administration of acetaminophen, AST level was significantly higher (p<0.05) in the no-garlic groups than in the garlic groups with no corresponding increase in CK levels, an indication that the AST in serum was predominantly from the liver and not from the muscles [1]. The lower level in the garlic group shows an enhanced cellular integrity of hepatocytes by garlic. An earlier study by Memudu AE, et al. [29], showed increased proliferative activity with retention of testicular integrity and increased testosterone levels in Sprague-Dawley rat upon ingestion of garlic cloves. By 2 days PAA, serum AST levels in the acetaminophen groups i.e., NG1 and G1 were higher than in the corresponding control groups NG2 and G2, respectively, with NG1 having significantly higher (p<0.05) level than G1. Inspite of these differences in serum AST levels, CK levels were not significantly different. According to Doneley B [1], an elevated AST level with normal CK level is an indication of hepatocellular damage. It is therefore apparent that there was leakage of AST from hepatocytes at this time, an indication of liver injury which was more severe in the No-Garlic group (NG1). By 14 and 21 days PAA, serum AST levels were significantly higher (p<0.05) in the No-Garlic groups (NG1 and NG2) than the Garlic groups (G1 and G2). However, serum CK levels were lower, the difference between NG1 and G2 being significant (p<0.05). Serum AST levels within each of the garlic and no-garlic groups showed no significant difference with respect to acetaminophen administration from 14 days PAA. It is known that drug-induced liver injury is an acute condition such that elevation of liver enzymes occurs soon after exposure to toxic doses [30], just as detected by Marmat S, et al. [31], 24 hours post administration of toxic doses of acetaminophen to broilers chicks. Thus the non-dectection of elevated AST levels in acetaminophen groups NG1 and G1 by 14 days PAA. It is worthy of note that experimental chickens that were separated from the No-Garlic/acetaminophen group (NG1) and placed on garlic-meal from one week PAA (NG1g), had significantly lower (p<0.05) level of AST when compared with NG1 by 14 and 21-days PAA. This signals the restorative ability of garlic on damaged liver cells as suggested by Memudu AE, et al. [29], Kodera Y, et al. [32], and Bozin B, et al. [33].
Serum creatinine levels were about equal in garlic and no-garlic groups pre-administration of acetaminophen. However, from 2 to 14 days PAA, levels were significantly higher (p<0.05) in acetaminophen groups NG1 and G1 than in their respective control groups NG2 and G2, and within the acetaminophen groups, NG1 had significantly higher (p<0.05) levels than G1. The result at 21-days PAA was also similar except that level in G1 was not significantly higher (p>0.05) than NG2 and G2. It is largely believed that creatinine has limited diagnostic value in avian species because a large proportion of creatine is excreted in urine before it is converted to creatinine such that levels in plasma are low [1]. However, the proportion left in plasma is usually excreted by glomerular filtration and reabsorbed in the tubules such that plasma concentrations are constant. Thus, reduction in glomerular filtration can lead to an increased creatinine concentration [34]. The increased serum creatinine levels recorded for the acetaminophen groups with or without garlic pre-treatment is believed to be due to acute kidney injury induced by the toxic dose of acetaminophen administered. The significantly higher (p<0.05) levels in no-garlic group NG1 than the garlic group G1 throughout the study period and the significantly lower (p<0.05) levels in NG1g group than NG1 group at 14 and 21 days PAA, signals reduced excretion by the kidney due to injury and a preservative or protective effect of garlic on the kidney tissue against toxic effects of acetaminiphen, respectively as opined by Memudu AE, et al. [29], Kodera Y, et al. [32], and Bozin B, et al. [33].
Serum proteins
Assay for serum proteins in the experimental chickens showed fairly uniform levels of total protein, albumin and globulin between garlic and no-garlic groups pre-administration of acetaminophen. However, from 2 to 21 days PAA, levels in the no-garlic/acetaminophen group NG1 were mostly significantly lower (p<0.05) than in the garlic/acetaminophen group G1. Also, the two garlic groups G1 and G2 almost consistently had higher serum protein levels than the no-garlic groups NG1 and NG2. Nogarlic/ acetaminophen group (NG1) constantly had significantly lower (p<0.05) levels than the garlic/acetaminophen group (G1). The liver is the site of production of plasma proteins, albumin, fibrinogen, lipoproteins and a variety of alpha and beta globulins [35,36]. According to Singh A, et al. [37], reduction in total protein concentration and particularly, albumin is an indication of hepatotoxicity as observed in chickens in NG1. However, the significantly higher concentrations recorded for garlic/ acetaminophen group G1 further emphasizes the protective effect of garlic on the liver. In addition, the comparatively higher serum albumin concentrations observed in the garlic/no-acetaminophen group G2 could be due to increased liver synthesis in response to availability of amino acids from the portal blood [35]. Oladele OA, et al. [11], had earlier reported increased absorptive capacity of the small intestine of broilers that were served garlic-meal as an additive in feed. Hypoproteinaemia as recorded for NG1 could also be due to kidney damage causing loss of globulins [35].
Clinical assessment
Clinical signs of toxicity were observed from 4 days PAA. Clinical assessment of the experimental chickens showed mild to marked dullness, anorexia and paleness in the NG1 (No- Garlic/acetaminophen) group from 5-to 14 days PAA, G1 (garlic/ acetaminophen) group was mildly anorexic and pale at only 8 days PAA while the NG1g (no-garlic/acetaminophen, subsequently placed on garlic-meal) showed improved anorexia and moderate dullness at 14 days PAA. An earlier study on the lethal doses of acetaminophen in broilers by Marmat S, et al. [19], reported dullness after four hours of administering 1500 and 1750 mg/kg body weight acetaminophen. According to Hochleithner M, et al. [36], clinical signs of hepatic failure in birds are variable and could range from mild inappetence and inactivity, to acute haemorrhage and death. Moderate watery faeces (polyuria) and jaundice were also observed in the NG1 group which were absent in other groups. Jaundice is an uncommon condition in avian species as they lack biliverdin reductase and glucuronyl transferase, the enzymes responsible for the conversion of biliverdin to bilirubin. As such, biliverdin is the primary bile pigment which gives the avian bile its characteristic green colour [1,36]. However, a study in broilers with gross hepatic bile duct lesions, traced the yellow colour of the pericardium and carcass to bilirubin [38]. Hochleithner M, et al. [36], explained that bacterial reduction may be responsible for the degradation of avian biliverdin to bilirubin in some cases.
Gross pathology
Swollen and congested liver, engorged gall bladder, swollen kidneys and petechial haemorrhages in skeletal muscles and joints surfaces were the gross pathology observed in acetaminophen groups with and without garlic pre-treatment. However, lesions were more severe in No-Garlic group (NG1) than in Garlic group (G1). Hepatomegaly as observed in chickens administered acetaminophen in this study is believed to be due to both inflammation because of innate response [39], and swelling of hepatocytes due to the cytotoxicity. These are known to cause occlusion of the biliary system and increase retention of bile i.e., cholestasis [1], as evidenced by engorged gall bladders observed in chickens in groups NG1 and G1. Haemorrhages observed in the groups of chickens that were administered acetaminophen could be due to inadequate production of clotting factors by the liver [1]. Swollen kidney as a gross expression of acetaminophen toxicity had earlier been reported by Elhabib EM, et al. [40]. The milder expression of these gross pathological lesions in chickens in the garlic/acetaminophen group (G1) once again indicates the protective effect of garlic on liver and kidney cells.
Histopathology
Histopathological lesions observed i.e., vacuolar degeneration and coagulative necrosis of hepatocytes, degeneration, and necrosis of tubular epithelium in kidneys and mononuclear cell infiltrations into both organs are evidence of hepato and nephrotoxicity induced by acetaminophen as earlier reported by Marmat S, et al. [31], Elhabib EM, et al. [40], as well as Blakely P, [41]. The observation of more mononuclear cells infiltrating the liver and kidneys of chickens in G1 than NG1 indicates an enhanced immune response in the garlic groups, more so that the cells were largely plasma cells. The NG1g group that were served garlic-meal from 8 days PAA and sampled at 14 days PAA showed milder necrosis and reduced inflammatory cells infiltration signaling tissue regeneration. In as much as the liver and kidney have innate capacity for regeneration post damage [36,42], the faster recovery by the NG1g group than the NG1 group is believed to be the effect of the garlic-meal feed additive.
Mechanism of toxicity and protection by garlic
The mechanism of toxicity induced by overdose of acetaminophen is via cytochrome P-450 mediated oxidative metabolism to a highly reactive intermediate metabolite, N-Acetyl-P-Benzoquinone Iminine (NAPQI), an electrophile and an oxidizing agent which is normally detoxified by glutathione peroxidase. However, when in excess this metabolite covalently binds with intracellular protein leading to cellular necrosis [43]. Some diseases of poultry species such as aflatoxicosis, simulate acetaminophen toxicity. Aflatoxin B1 is known to be strongly hepatotoxic and carcinogenic [44]. It undergoes a biological change into several metabolites which bind DNA and RNA, reduce protein synthesis, and decrease both antibody-mediated and cell-mediated immunity. Liver, kidney, and spleen becomes enlarged, and fat accumulates in liver cells as clear vacuoles in high-dose exposure. Small haemorrhages also occur due to decreased synthesis of clotting factors [45]. Yuns AW, et al. [44], reported that cytochrome P-450 enzymes in the liver and other tissues convert aflatoxin B1 to metabolites which covalently bind DNA and other intracellular proteins causing toxicity and cancer.
The protective effect of garlic on cells is believed to be via its antioxidant components i.e., flavonoids and sulpur-containing compounds i.e., diallyl sulfides, trisulfide and allylcysteine [32,33]. A study by Jeong JH, et al. [46], investigated the antioxidant activities and neuron-like PC 12 cell protective effects of solvent fractions from aged garlic extracts. They reported that ethyl acetate fractions showed the highest amyloid beta (Aβ) protein radical scavenging activity and malondialdehyde inhibitory effect and concluded that these fractions showed protection against Aβ- induced neurotoxicity. Butt MS, et al. [47], had earlier reported that garlic components or formulations can scavenge free radicals, protect membranes from damage and maintain cell integrity. Also, Memudu AE, et al. [29], demonstrated the retention of testicular integrity and testosterone levels because of ingestion of garlic cloves by Sprague-Dawley rats and inferred that garlic has proliferative and restorative potentials on cells. The chemo-preventive potential of garlic has been attributed to the presence of several bioactive organosulfur compounds which might act as antioxidants [48].
Thus, the inclusion of garlic in the feed of chickens in this study which enhanced antibody response to Newcastle disease vaccination, reduced serum AST and creatinine levels, increased serum protein production and reduced clinical signs and pathological lesions associated with toxic dose of acetaminophen as well as the reversal of these toxic effects on the liver and kidney, shows the potential of garlic in protection against hepatorenal damage or injury. It is therefore recommended that the inclusion of this garlic-meal in feed for chickens at a rate of 0.25% could be beneficial in protection against diseases and conditions that impair the health and functions of the liver and kidney.
Ethics governing the use and conduct of experiments on animals were strictly observed, and the experimental protocol was approved by the Animal Care and Use Research Ethics Committee of the University of Ibadan (UI-ACUREC/App/2016/013).
The authors appreciate the assistance provided by Mr. Ini Akpan in caring for the experimental chickens and Mrs. Iyabo A. Adetiba for providing laboratory assistance.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Oladele OA, Nottidge HO, Esan OO, Bakre AA (2023) Garlic (Allium sativum Linn) Mitigates Experimentally Induced Hepatotoxicity and Nephrotoxicity in Commercial Chickens. J Clin Toxicol. 13:524
Received: 19-Dec-2022, Manuscript No. JCT-22-21021; Editor assigned: 21-Dec-2022, Pre QC No. JCT-22-21021 (PQ); Reviewed: 04-Jan-2023, QC No. JCT-22-21021; Revised: 11-Jan-2023, Manuscript No. JCT-22-21021 (R); Published: 19-Jan-2023 , DOI: 10.35248/2161-0495.23.13.524
Copyright: © 2023 Oladele OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.