Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2016) Volume 5, Issue 1
Ulcer is a common gastrointestinal disorder, irregular food habits, stress, and so forth. Peptic ulcers are a broad term that includes ulcers of digestive tract in the stomach or the duodenum. The formation of peptic ulcers depends on the presence of acid and peptic activity in gastric juice plus a breakdown in mucosal defenses. A number of synthetic drugs are available to treat ulcers. But these drugs are expensive and are likely to produce more side effects when compared to herbal medicines. The ideal aims of treatment of peptic ulcer disease are to relieve pain, heal the ulcer, and delay ulcer recurrence. In this present study was carried out to evaluate the gastro protective effect of flavonoid glycoside (G1) from the flowers of Guttarda Speciosa against ethanol induced gastric ulcer in rats.
Keywords: Pepticulcer; Gastric juice; Guttarda speciosa; Flavonoid glycoside; Male albino rats
Peptic ulcer (encompassing gastric ulcer and duodenal ulcer) is a major health hazard both in terms of morbidity and mortality. Peptic ulcer disease affect a large portion of the world population and are induced by several factors, including stress, smoking, nutritional deficiencies, and ingestion of non-steroidal anti-inflammatory drugs [1]. The pathophysiology of these ulcers involves an imbalance between offensive (acid, pepsin, and Helicobacter pylori) and defensive factors (mucin, prostaglandin, bicarbonate, nitric oxide and growth factors). Today, there are two main approaches for treating peptic ulcer. The first deals with reducing the production of gastric acid and the second with re-enforcing gastric mucosal protection [2].
Peptic ulcer disease and gastric dyspepsia-associated with chronic use of therapeuticals such as non-steroidal anti-inflammatory drugs (NSAIDs) and anticancer agents are the two major causes that adversely affect the life quality. Presently used antisecretory agents like proton pump inhibitors may represent a key option in peptic ulcer therapy [3] but their prolonged use seems to be associated with high incidence of hip fractures. NSAIDs induced gastropathy remains a major clinical problem [4] which has not been solved through the introduction of selective inhibitors of cyclooxygenase-2 (COX-2) due to cardiac side effects [5]. The World Health Organization (WHO) has estimated that there are about 2 billion people worldwide who consume alcoholic beverages and 76.3 million with diagnosable alcohol use disorders. Alcohol consumption is an important factor to induce the gastric ulcer.
Among the various organ systems that mediate alcohol’s effects on the human body and its health, the gastrointestinal tract plays a particularly important role. The alcohol absorption into the bloodstream occurs throughout the gastrointestinal tract and its direct contact with the mucosa can induce numerous metabolic and functional changes. These alterations may lead to marked mucosal damage, which can result in a broad spectrum of acute and chronic diseases, such as gastrointestinal bleeding and ulcers.
Chari et al. relate that intravenous, oral, and intra-gastric alcohol at a concentration of up to 5% increases acid secretion principally by stimulating the secretion of gastrin and to a lesser extent by a direct effect on the parietal cells. On the other hand, an alcohol concentration of higher than 5% has no effect on gastric acid secretion. Also, oxidative stress and depletion of non-protein sulfhydryl’s concentration, modulation of nitric oxide system and reduction of gastric mucosal blood flow frequently underlie the development of gastric lessions. The decreased formation of prostaglandins might also play a role in alcohol-induced mucosal injury, while other studies have indicated that an alcohol-dependent increase in the production of leukotrienes also might contribute to the development of alcohol-induced damage. It is important emphasize that changes induced by short-term exposure to alcoholic beverages are rapidly reversible while prolonged alcohol exposure leads to progressive structural mucosal damage). Ethanol consumption has been shown to be a major cause of gastric ulcer. The ethanol-induced increase in oxidative stress can culminate in tissue damage in various organs.
Oxidative stress and depletion of anti-oxidants have been considered a crucial step in alcohol-induced mucosal damage and so they have been widely investigated in a number of studies (Arafa & Sayed-Ahmed). Ethanol treatment induces intracellular oxidative stress and produces mitochondrial permeability transition and mitochondrial depolarization, which precede cell death in gastric mucosal cells. Thus, considering that ethanol is involved in the formation of oxidative stress generated extracellular and/or intra-cellular, the cyto-protective role of anti-oxidants in the prevention and healing of gastric lesions has also been widely investigated.
Therefore, the protection of tissue damage has become a rational approach in preventing ethanol-induced toxicity. In recent years, there is an active search to discover novel and alternative agents useful to combat gastric dyspepsia, and peptic ulcer disease. Worldwide interest in natural products as preventive and therapeutic agents has led to a greater appreciation of the rich heritage of traditional systems of medicine. Dietary and lifestyle modifications are the basis of Ayurveda medicine, with herbal formulas rounding out therapeutic programs. Ayurveda formulas contain many balancing herbs offering a high degree of safety and efficacy. The present study was carried out to evaluate the gastroprotective effect of flavonoid glycoside G1isolated from the flowers of Guttarda speciosa against ethanol induced gastric ulcer in rats.
Animals
Male albino rats of Wistar strain approximately weighing 160- 180g were used in this study. They were healthy animals purchased from the Indian Institute of Science, Bangalore. The animals were housed in spacious polypropylene cages bedded with rice husk. The animal room was well ventilated and maintained under standard experimental conditions (Temperature 27 ± 2°C and 12 hr light/dark cycle) throughout the experimental period. All the animals were fed with standard pellet diet and water were provided ad libitum. They were acclimatized to the environment for one week prior to experimental use. The animal feed composition is crude protein (22.3%), crude oil (4.01%), crude fibre (4.02%), Ash (8.02%) and sand silical (1.02%).
Preparation of plant extract
Fresh flower of Guttarda speciosa collected from kumbakonam ,Tanjore district, during December were extracted with 90% methanol(3X500) under reflux. The alcoholic extract was concentrated in vacuo and the aqueous concentrate was successively fractionated with benzene (3X250), ether (3X250) and ethyl acetate (4X250). The ether fraction did not yield any isolable material ethyl acetate fraction alone was taken for study.
Ethanol-induced gastric ulcer
Animals were randomly divided into four groups each of 6 rats. Gastric lesions were induced with ethanol (96%) at a dose of 0.2 ml/ animal [6]. Group I served as control group received saline orally. Group II animals served as ulcerogenic group received ethanol orally. Group III animals received aqueous extract of G1 at a dose of 500 mg/ kg orally. Group IV was orally administered 20 mg/kg (ip) Omeperazole as a standard drug. Forty-five minutes after treatment with plant extract and standard drug, each animal was given orally 0.2 mL of ethanol (96%). and they were sacrificed 30 min later. After 30 min, the rats were sacrificed and the stomach was removed. The gastric content was collected and centrifuge for 5 min at 2000 x g and the supernatant was separated. The volume, pH, and total acidity, free acidity of gastric fluid were determined.
Measurement of ulcer index
The stomachs were excised and were examined for hemorrhagic lesions in glandular mucosa. Immediately after the animals were sacrificed, their stomachs were dissected out, cut sqaalong the greater curvature and the mucosa were rinsed with cold normal saline to remove blood contaminant, if any. The surface area of each lesion was counted and scored as described by Tan et al. [7] (1996). The ulcer index for each rat was taken as the mean ulcer score. The percentage of ulcer protection (%UP) was calculated as described by Nguelefack et al. [8] using the following formula:
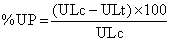
Where ULc=Ulcer lesion in control group and ULt=Ulcer lesion in treated groups.
Biochemical estimations
Determination of gastric juice volume and pH: The volume and pH of centrifuged gastric juice were measured by pipette and digital pH meter. The volume was expressed as ml [9].
Determination of total and free acidity: The total and free acidity were determined by titrating with 0.01N NaOH using phenolphthalein and Topfer’s reagent or methyl orange [10].
Reagents
1. 0.01N NaOH
2. Phenolphthalein
3. Topfer’s reagent or methyl orange
Procedure
Pipette 1 ml of filtered gastric contents into a small beaker, add 2 to 3 drops of Topfer’s reagent or methyl orange and titrate with 0.01 N NaOH until all trace of the red colour disappears and the colour is yellowish orange. Note the volume of alkali added that indicate free acidity. Then add 2 or 3 drops of phenolphthalein and continue titrating until a definite red tinge reappears. Note the total volume of alkali added that indicate total acidity.
The results expressed as Meq/l.
Statistical analysis
Values were expressed as mean ± SD for six rats in the each group and statistical significant differences between mean values were determined by one way analysis of variance (ANOVA) followed by the Tukey’s test for multiple comparisons. The results were statistically analyzed by Graphpad Instat Software (Graphpad Software, San Diego, CA, USA) version 3 was used and p<0.05; p<0.01 and p<0.001 were considered to be significant.
Antiulcer activity
The effect of orally administered G1 on gastric damage induced by absolute ethanol is shown in Table 1. It was observed that increase the ulcer lesion in ulcer control rats. Significant reduction in ulcer lesion was observed in treatment with-G1. It is significant to note that increased the volume, total acidity and free acidity and decreased pH of gastric juice were observed in ulcer control rats as compared to normal rats. Administration of G1 decreased the volume, total acidity and free acidity and increased pH of gastric juice were observed as compared to control rats. Animal groups treated with the G1 (500 mg/kg,) exhibited a reduction of gastric damage against ethanol-induced gastric ulceration. The percentage of ulcer protection was 77.87% for G1and 77.80% for standard. Omeperazole, the positive control included for the study also offered significant protection (77.87%) against ethanol induced gastric ulcer (Table 1). The G1 percentage of inhibition was higher than that of standard.
| Groups | pH | Volume | Free acidity | Total acidity |
GastricUlcer lesion (No.) | % of Ulcer protection |
|---|---|---|---|---|---|---|
| Group I | 2.4 ± 0.16 | 1.3 ± 0.09 | 220 ± 15.4 | 260 ± 18.2 | 1± 0.07 | -- |
| Group II | 1.3 ± 0.11 a | 3 ± 0.21 a | 320 ± 22.4 a | 360 ± 25.2 a | 9± 0.63 a | -- |
| GroupIII | 2.2 ± 0.15 b | 1.6 ± 0.11 b | 180 ± 12.8 b | 240 ± 16.8 b | 2 ± 0.14 b | 77.87 |
| Group IV | 2.6 ± 0.16 b | 0.6 ± 0.04 b | 120 ± 8.4 b | 180 ± 12.6 b | 1 ± 0.08 b | 88.90 |
Table 1: Effect of G1 on pH, Volume, Acidity, Ulcer lesion in control and experimental rats.
Peptic ulcer is defined as disruption of the mucosal integrity of the stomach and/ or duodenum leading to a local defect or excavation due to active inflammation.
Several experimental models were used to assess the antiulcerogeinic activity of test drugs in rats. They include Cold restraint stress, Pyloric ligation, Aspirin, Indomethacin, Cysteamine, ethanol etc. Mucus, which continuously coats over the gastric mucosa, is well known as a “mucous barrier” to prevent the injury of luminal acid, bacteria and noxious agent’s injuries. Mucus might implicate in scavenging oxygen derived free radicals. Mucus glycoproteins and lipids bound to mucin might involve in the antiradical process.
In the present investigation, it has been demonstrated that G1 can significantly enhance gastric mucus secretion while reducing the acidity of the gastric juice in rats. Gastric mucus is an important protective factor for the gastric mucosa and it is capable of acting as an antioxidant agent and reducing mucosal damage mediated by oxygen free radicals .However, the protective properties of the mucus barrier depend not only on the gel structure but also on the amount or thickness of the layer covering the mucosal surface.
G1 possesses marked gastro-protective properties as evidenced by its significant inhibition of the formation of gastric lesions (in terms of length and number) induced by absolute ethanol. In the present study, the decrease in volume of the gastric juice and concomitant decrease in the acidity and increase in pH proving the antiulcer activity of G1 and this result complements the earlier findings. Further evidenced by the reduced the edema formation and epithelial lifting were observed in morphometric study.
Administration of G1 significantly increased the amount of mucus produced by the rat gastro mucosa compared to their respective controls. Therefore, the enhanced mucus secretion after administration of G1 may help to protect against the absolute ethanol induced damage by preventing the action of acid on the stomach mucous epithelium. It is also well known that prostaglandins synthesized in large quantities by the gastrointestinal mucosa can prevent experimentally induced ulcers by ulcerogens. Thus, when the ulcer lesions are induced by absolute ethanol, the cyto-protective effect of the antiulcer agent can mediate through endogenous prostaglandins. Therefore, it can be thought that G1 may stimulate the secretion of prostaglandin or possess prostaglandin like substances.
Gastroprotective role for G1 against gastric mucosal damage induced by ethanol were investigated in the present study. Ethanol induced gastric ulcer rat’s shows that increased gastric volume, acidity and depleted pH. The observed gastroprotection is possibly mediated to a major extent by a gastric mucosal secretion mechanism as the G1 were able to restore the increased volume, acidity and depleted pH by ethanol almost towards normal levels seen in control. This is further evidenced by morphometric study. Other complementary mechanisms may include the activation of capsaicin-sensitive gastric afferents, stimulation of endogenous prostaglandins and nitric oxide, and opening of K+ATP channels. These combined effects are likely to be accompanied by an increase in gastric microcirculation. The percentage of ulcer protection was 77.78% for G1 and 77.80% for standard. Omeperazole, the positive control included for the study also offered significant protection (77.80%) against ethanol induced gastric ulcer. The G1 percentage of inhibition was higher than that of standard. Hence, the gastro-protective effect observed in the present study which might have been due to phytochemical such as flavonoid glycoside.