Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2020)Volume 9, Issue 7
Background: Obesity and regional fat distribution are among the strongest risk factors for obstructive sleep apnea (OSA) in the general population. People living with HIV (PLHIV) may be at increased risk of OSA because of their distinct body fat composition. This study examined the association of body fat composition with the risk of OSA in PLHIV.
Methods: We conducted a cross-sectional prospective study at a tertiary HIV care unit, recruiting 400 PLHIV [female: 317, 79.2%; median age of 44 years; median year of HIV duration of 5 years]. Participants were surveyed using standardized instruments to assess the risk of OSA, sleep duration and, sleepiness. Body fat composition was evaluated with anthropometric measurements and bioelectric impedance
Results: Sixty-three of the participants (15.8%; 95% confidence interval [CI], 12.4%–19.8%) had risk of OSA, while 24 (6.0%) of them had risk of OSA and excessive day time sleepiness and 30 (7.5%) had risk of OSA with concomitant short sleep duration. BMI and markers of central obesity were significantly associated with risk of OSA in females but not in males. Waist circumference, Waist to hip ratio, Visceral fat level performed similarly in predicting the risk of OSA in females with Area Under the Curve (AUC) of 0.668, 95% CI (0.613, 0.719); 0.704, 95% CI (0.651, 0.754); 0.663, 95% CI (0.608, 0.715) respectively, with VFL having the best accuracy.
Conclusions: The risk of OSA among PLHIV was associated with measures of visceral adiposity in females and not in males.
Body fat percentage; Visceral fat; Sleep duration; Sleepiness; Obstructive sleep apnea; HIV/AIDS
Obstructive sleep apnea (OSA) is a markedly prevalent sleep disorder globally. It spans across age groups and gender [1], and is associated with long-term cardiovascular, metabolic and neurocognitive health consequences [2,3]. Aside from these, the public health and economic consequences of undiagnosed and untreated OSA are enormous [4,5]. The risk of OSA has been extensively reported among older adults [6], persons with hypertension [7], and diabetes mellitus [8], as well as in other population [9,10,11]. However, data are sparse on the risk of OSA among people living with HIV (PLHIV) [12,13].
PLHIV may have an increased risk of OSA. Abnormal body fat deposition and increased weight gain from antiretroviral medications may increase their risk [13,14]. Inflammation related to HIV disease itself may increase the risk of OSA. Also, the availability of effective therapy has made HIV infection a chronic and aging condition with improved life expectancy and increased predisposition to OSA [14,15].
Polysomnography (PSG) remains the gold standard for diagnosis of OSA [16], however, it is labor and resource-intensive and not readily available. For instance, in Nigeria, there are only two sleep laboratories that serves a population of more than 200 million citizens. Due to the increasing prevalence of undiagnosed OSA and its associated comorbidities, screening questionnaires are needed for prompt prediction of OSA. These screening procedures such as questionnaires can determine the risk of OSA, triage based on the need for urgent PSG, and facilitate the management of those identified in clinical settings [17,18].
The most common and consistent factor included in these screening questionnaires is obesity which is not only the most well-documented but also the most important reversible risk factor [2,19]. However, depending only on a general measure of obesity, such as body mass index(BMI) has some limitations, as it may not truly represent regional fat distribution which is more implicated in the pathogenesis of OSA [20,21]. Other anthropometric measurements such as neck circumference (NC), waist circumference (WC), hip circumference (HC) and its derived ratios, have been more associated with OSA (21) in general population. More so, in PLHIV, BMI might not be associated with OSA [22].
To date, studies detailing the risk of OSA among PLHIV and its association with multiple indices of obesity and body fat composition are lacking in sub-Saharan Africa. In this study, our goal was to assess the risk of OSA and investigate which body fat composition measurements predict the risk of OSA among PLHIV
Study design and participants
We conducted a cross-sectional study of 400 PLHIV receiving care at the HIV clinic, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria from July to December 2017. The HIV clinic is a prototypical, government-run HIV clinic, provides holistic HIV care for South-Western people in Nigeria. Ithas 1407 registered PLHIV. Participants were included if they were 18 years or older and willing to participate in the study. We excluded those who had craniofacial abnormalities and any acute medical condition that could affect their ability to complete the study questionnaire
The study was approved by the Ethics and Research Committee of ObafemiAwolowo University Teaching Hospitals Complex with protocol number ERC/2016/02/01. Written informed consents were obtained from all the participants.
A sample size of 381 was calculated using a single proportion sample size formula for estimating the minimum sample size in descriptive health studies, with estimated prevalence of OSA risk of 43.6% from previous study [23]; at 5% margin of error and 95% confidence interval. On each clinic day, the trained research assistants interviewed 30-35 participants selected by simple random sampling (lottery method) in the clinic.
Assessment of OSA risk, sleep duration and sleepiness
We assessed OSA risk using the STOP-BANG (Snoring, Tiredness, Observed apnea, high blood Pressure, BMI >35 kg/m2, Age >50 years, Neck circumference >40 cm, male Gender) questionnaire, which is a simple and widely used OSA risk screening tool. It has 8 items and each positive item scores 1 point; a score ≥ 3 out of 8 is considered intermediate to high risk for OSA. It has a high sensitivity 84% in detecting any sleep apnea [18,24,25]. As our goal was to determine the anthropometric predictors of OSA risk, we excluded BMI and neck circumference from STOPBANG questionnaire to prevent the use of these variables as both dependent and independent factors. We estimated subjective habitual sleep duration by using the first 4 items of the Pittsburgh Sleep Quality Index. Participants that slept less than 7 hours per day were classified as having short sleep duration (SSD) [26]. We also quantified excessive daytime sleepiness (EDS) by using the Epworth Sleepiness Scale (ESS). Patients with ESS score of 10 or more were considered to EDS [27].
Anthropometric and body fat measurements
Interviewers were trained to measure anthropometric measurements using the protocol established in the 3rd National Health and Nutrition Examination Survey [28]. Briefly, height was measured with a wall-mounted stadiometer to the nearest 0.1cm and body weight recorded to the nearest 0.1 kg using the Omron body scale and BMI was then calculated in kg/m2. The measurement of the neck, waist, and hip circumferences were recorded to the nearest 0.1cm using a non-elastic tape. The Omron BF-511 (50kHz, 500μA, Tokyo, Japan) body composition analyzer was used to measure body fat composition. Body Fat percentage (BF%) was recorded to the nearest 0.1% and visceral fat content was expressed in 30 levels. It has been demonstrated that the VFL determined by bioelectrical impedance analysis(BIA) correlated closely with the VF area determined by computed tomography, for example, visceral at level 10, corresponded to a VF area of 100 cm2 [29]. All the measurements were taken by the trained research staff and regularly supervised by the lead investigator to ensure quality control and adherence to the standardized protocol.
Other co-variates
These included demographic variables, co-morbid disease conditions, HIV disease and clinical parameters (including time since diagnosis, prescribed antiretroviral medications, current CD4 count which were abstracted from their medical charts, and fatigue). Others are social parameters such as smoking status, alcohol consumption, and employment status.
Statistical analyses
Statistical analyses were performed with IBM-SPSS software V.20 (IBM, Armonk, New York, USA) and MedCalc statistical software V.18.2 (MedCalc software bvba, Ostend, Belgium). Participants’ demographics and clinical characteristics were described using frequencies and percentages for categorical variables. The anthropometric measurements were not normally distributed and so, were expressed as median values with interquartile ranges (IQR). Normality was tested with the Shapiro-Wilk test. Differences in clinical characteristics between the intermediate to high risk and the low risk groups were tested with the Chisquare test or the Fisher exact test for categorical variables, and with unpaired Wilcoxon rank-sum test (Mann–Whitney U test) for anthropometric variables. Because anthropometrics and body fat measures are influenced by gender, subsequent analyses to detect associations of body fat composition with the risk of OSA were stratified by gender. To explore the association between OSA risk and each anthropometric measure, multiple logistic regression analyses were performed individually using OSA risk as the dependent factor and one anthropometric variable (BMI, WC, BF%, Visceral fat level, HC, WHtR) at a time as independent covariate, controlling for age, educational level, CD4 count, years of HIV diagnosis and comorbidities. The outcomes for receiveroperating characteristic ROC Curves were defined in 3 ways: (1) STOP with age and gender score ≥3 (2) STOP score with age and gender ≥3 with concomitant ESS ≥10 (3) STOP with age and gender with concomitant sleep duration <7hours). For comparison of the indices, the area under curves (AUC) and Youden index were performed to determine the optimal cut-off point for the individual anthropometric indices for predicting the risk of OSA in females. A p-value of <0.05 was considered significant and all the tests were two-tailed.
Characteristics of the participants
We assessed 400 HIV-infected persons with a median age of 44 years (interquartile range [IQR], 37–52 years), 79.2% were female and 71.6% were married. The median time since HIV diagnosis was 5 years (interquartile range [IQR], 2–8 years); the median duration on antiretroviral therapy (ART) was 4 years (IQR, 2-7 years); 7.2% had a current CD4+ cell count below 200 cells/μl, and 26.2% had a current CD4+ cell count between 200 and 499 cells/μl. The majority (95%) of the participants had never smoked and only 2.5% reported alcohol use (Table 1).
| Variables | Total participants N=400 | Risk of OSA N=63 | No of Risk of OSA N=3I7 | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| Median age | 44.0 (37.0-52.0) | 53.0 (48.3-.57.0) | 42.0 (36.0-49.0) | <0.001 |
| <30 | 23(5.7%) | 2(3.2%) | 21(6.2%) | <0.001 |
| 30-39 | 110(27.5%) | 3(4.8%) | 107(31.8%) | |
| 40-49 | 139(34.7%) | 13(20.6%) | 126(37.4%) | |
| ≥ 50 | 128(32.0%) | 45(71.4%) | 83(24.6%) | |
| Gender | ||||
| Female | 317(79.2%) | 39(61.9%) | 278(82.5%) | 0.002 |
| Male | 83(20.8%) | 24(38.1%) | 59 (17.5%) | |
| BMI (kg/m2) | ||||
| Median BMI | 22.5(19.9-26.6) | 24.6(20.8-28.0) | 22.0(19.6-25.7) | 0.014 |
| <25 | 264(66.0%) | 33(52.4%) | 231(68.5%) | |
| 25-29.9 | 105(26.2%) | 25(29.7%) | 80(23.7%) | |
| ≥30 | 31(7.8%) | 5 (7.9%) | 26(6.5%) | 0.027 |
| Educational level | ||||
| No education or Primary school | 89(22.2%) | 17(26.9%) | 72(22.7%) | |
| Secondary school | 183(45.8%) | 25(39.7%) | 158(49.6%) | 0.319 |
| Tertiary institution | 128(32.2%) | 21(33.4%) | 107(31.7%) | |
| Marital status | ||||
| Single | 25(6.2%) | 2(3.2%) | 23(6.8%) | 0.672 |
| Married | 287(71.7%) | 47(74.6%) | 240(71.2%) | |
| Divorced | 20(5.0%) | 10(5.9%) | 16(4.7%) | |
| Widowed | 68(17.0%) | 10(15.9%) | 58(17.2%) | |
| Employment | ||||
| Yes | 305(76.2%) | 51(81.0%) | 254(75.4%) | 0.334 |
| No | 95(23.7%) | 12(26.6%) | 83(24.6%) | |
| Smoking | ||||
| Never smoked | 381(95.2%) | 59(93.7%) | 322(95.5%) | |
| Ever smoked/current smoker smsmokersmokerssssmsmoking | 19(4.8%) | 4(6.3%) | 15(4.5%) | 0.164 |
| Alcohol Consumption | 10(2.5%) | 1(1.6%) | 9(2.7%) | 0.783 |
| Duration of HIV (years) ((9(diagnosis(years) | 5.0(2.0-8.0) | 5(3.0-8.0) | 5(2.0-8.0) | 0.293 |
| Cart | ||||
| No therapy | 13(3.2%) | 2(3.2%) | 11(3.3%) | |
| AZT/3TC/NVP | 192(48.0%) | 31(49.2%) | 161(47.8%) | 0.739 |
| TDF/3TC/EFV | 179(44.7%) | 26(41.3%) | 153(45.5%) | |
| Other combination | 16(4.0%) | 4(6.3%) | 12(3.2%) | |
| CD4 count cells/µL | ||||
| <200 | 29(7.2%) | 4(6.3%) | 25(7.4%) | |
| 200-499 | 105(26.2%) | 12(19.0%) | 93(27.6%) | |
| ≥500 | 266(66.5%) | 47(74.6%) | 219(65.0%) | 0.317 |
| Comorbidities | ||||
| Any comorbidity | 124(31.1%) | 34(50.0%) | 50(26.7%) | <0.001 |
| Hypertension | 67(16.8%) | 27(42.9%) | 40(11.9%) | <0.001 |
| Diabetes mellitus | 6(1.5%) | 2(3.2%) | 4(2.1%) | 0.285 |
| CVA | 9(2.2%) | 2(3.2%) | 7(2.1%) | 0.938 |
| PTB | 32(8.0%) | 6(9.5%) | 26(7.7%) | 0.635 |
| Peptic ulcer disease | 41(10.2%) | 9(14.3%) | 32(9.5%) | 0.259 |
| Other comorbidities | 14(3.5%) | 5(7.9%) | 9(2.7%) | 0.062 |
| Presence of fatigue | 67(16.8%) | 12(19.4%) | 55(16.3%) | 0.552 |
| ESS ≥10 | 152(38.2%) | 24(38.1%) | 128(38.0%) | 0.988 |
| Sleep duration <7hrs | 154(38.5) | 30(47.6) | 124(36.8) | 0.106 |
Data are shown as medians (interquartile range), or numbers (percentages of respondents). Mann Whitney test for continuous variables; pearson chi-square for categorical variable, BMI: body mass index, ESS-Epworth sleepiness scale, cART-combination antiretroviral therapy, CVA: cerebrovascular accident, PTB: pulmonary tuberculosis, AZT-Zidovudine, 3TC-Lamivudine, NVP-Nevirapine, TDF-Tenofivir, EFV-Efavirenz. OSA risk; participants with intermediate and high risk, No risk of OSA; participants with low risk
Table 1: Sociodemographic and clinical characteristics of the participants.
Prevalence of OSA risk
Of the 400 participants, 63 (15.8.%; 95% confidence interval [CI], 12.4%–19.8%) were classified as having intermediate and high risk of OSA (stop-bang score ≥ 3 when BMI and NC were excluded from the criteria). Including BMI and NC in the criteria, 74 (18.5.%; 95% confidence interval [CI], 14.9%–22.7%) of them were classified as having intermediate and high risk of OSA. The prevalence of high risk of OSA according to modified stop bang criteria was 6.5% (4.4%-9.5%). Using a composite variable of STOPAG with EDS; 24(6.0%; 95% CI; 4.0%- 8.9%), and the composite variable of STOPAG with SSD of <7 hours, 30 (7.5%; 95% CI, 5.2%- 10.6%) were classified as having OSA risk respectively (Figure 1). There were significant differences in the prevalence of OSA risk in males and females as assessed by STOP-BANG, STOPAG and modified STOP-BANG score (39.4% vs14.2% p<0.001, 28.9% vs12.3% p<0.008, 16.9% vs 3.9%p<0.001) respectively. However, there were no statistically significant differences between males and females, when the composite score of STOPANG with EDS or SSD (7.2% vs 5.7% p=0.596; 12.2% vs 6.3%p=0.077) were assessed.
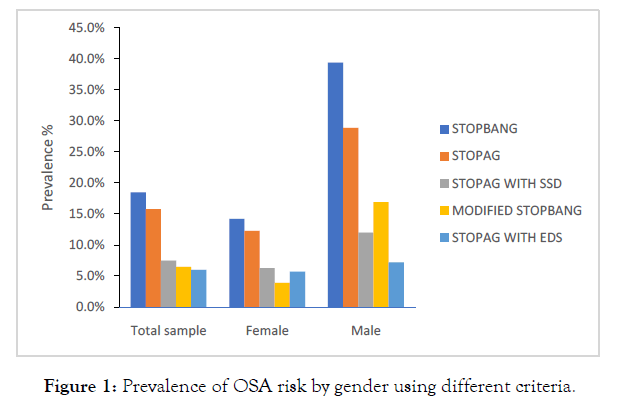
Figure 1: Prevalence of OSA risk by gender using different criteria.
Factors associated with OSA risk
Participants who were at risk for OSA were more likely to be older (53 years vs 42 years p<0.001), male (38.1% vs 17.5%; p=0.002), overweight (median BMI 24.6 vs 22.0; p=0.014) and had other comorbidities (50.0% vs 26.7%; p<0.001). However, other socio-demographics and clinical variables did not significantly. Smoking and alcohol consumption showed no association with the risk of OSA, sleepiness and short sleep were also not associated with OSA risk.
Of the anthropometric measurement, waist to hip ratio predicted OSA risk in males. In females, significant differences existed in median body mass index BMI (26.0 vs. 22.4; p=0.002), waist circumference WC (91.0 vs. 82.0; p=0.001). BF% (37.0% vs.30.5%; p=0.005), and VFL (8.0 vs 5.0; p<0.001) for those at risk and those who were not respectively (Table 2).
| Anthropometric | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| Variables | OSA risk | No OSA risk | P value | OSA risk | No OSA risk | P value | |
| Weight (kg) | 69.0(59.0-74.0) | 60.0(52.0-70.0) | 0.005 | 64.0(54.3-73.5) | 66.0(57.0-74.0) | 0.462 | |
| Height (m) | 1.62(1.59-1.66) | 1.62(1.59-1.67) | 0.809 | 1.71(1.65-1.76) | 1.75(1.70-1.80) | 0.047 | |
| BMI (kg/m2) | 26.0(21.0-28.5) | 22.4(19.2-26.0) | 0.002 | 21.6(19.1-24.9) | 21.6(19.1-25.0) | 0.651 | |
| BF% | 37.0(29.0-42.6) | 30.5(24.2-38.7) | 0.005 | 18.1(12.3-24.7) | 18.7(14.0-23.1) | 0.921 | |
| VFL | 8.0(5.0-9.0) | 5.0(3.0-7.0) | <0.001 | 5.0(3.0-9.0) | 4.0(2.0-7.0) | 0.188 | |
| WC (cm) | 91.0(81.0-96.0) | 82.0(76.0-91.0 | 0.001 | 84.5(77.3-94.0) | 83.0(76.0-89.0) | 0.418 | |
| HC (cm) | 105.0(93.0-113.0) | 97.5(91.0-105.3) | 0.005 | 93.5(86.3-98.8) | 93.0(88.0-99.0) | 0.918 | |
| WHR | 0.89(0.82-0.92) | 0.84(0.81-0.89) | 0.031 | 0.93(0.88-0.97) | 0.88(0.85-0.90) | 0.024 | |
| NC | 34.0(32.0-35.0) | 33.0(32.0-33.0) | 0.258 | 36.5(35.0-38.0) | 37.0(35.0-39.0) | 0.460 | |
| WHtR | 0.55(0.57-0.60) | 0.51(0.47-0.56) | 0.001 | 0.50(0.46-0.54) | 0.48(0.44-0.52) | 0.074 | |
Data are expressed as medians (interquartile range), or numbers (percentages of respondents). P-value were calculated based on Mann Whitney test for continuous variables; and Person chi-square for categorical variable, BMI: body mass index, WC: waist circumference, HP: Hip circumference, NC: neck circumference, WHtR: waist to height ratio, WHR: waist to hip ratio, BF%:Body fat percentage, VFL: visceral fat level.
Table 2: Anthropometric measurements and OSA risk stratified by gender.
After adjusting for age, educational status, alcohol consumption, smoking, duration of HIV, cART,CD4 counts and any comorbidities, BMI [Odd ratio (OR), 1.080; 95% confidence interval (CI), 1.010–1.156; p=0.025)], WC (OR, 1.038; 95% CI, 1.006–1.072; p=0.021), BF% (OR, 1.059; 95% CI, 1.017–1.102; p=0.005), VFL (OR, 1.231; 95% CI, 1.081–1.402; p=0.002) and WHtR (OR, 1.062; 95% CI, 1.010–1.117; p=0.019) were associated with the risk of OSA in females (Table 3).
| Female | Male | |||
|---|---|---|---|---|
| Anthropometric measurements | OR (95% CI) | P Value | OR (95%Cl) | P Value |
| BMI (kg/m2) | 1.080 (1.010-1.156) | 0.025 | 1.071 (0.891-1.286) | 0.465 |
| WC (cm) | 1.038 (1.006-1.072) | 0.021 | 1.017 (0.954-1.080) | 0.580 |
| BF% | 1.059 (1.071-1.102) | 0.005 | 1.044 (0.948-1.151) | 0.380 |
| VFL | 1.231 (1.081-1.402) | 0.002 | 1.126 (0.945-1.341) | 0.185 |
| HC (cm) | 1.024 (0.996-1.053) | 0.096 | 0.978 (0.911-1.049) | 0.531 |
| WHR | 1.052 (0.990-1.118) | 0.103 | 1.102 (0.969-1.228) | 0.077 |
| WHtR | 1.062 (0.010-1.117) | 0.019 | 1.106 (0.990-1.235) | 0.074 |
All analyses were adjusted for educational level, years of HIV, smoking, alcohol, cART, CD4 counts and comorbidities. BMI: body mass index, WC: waist circumference, HP: Hip circumference, NC: neck circumference, WHtR: waist to height ratio, WHP: waist to hip ratio, BF%: Body fat percentage, VFL: visceral fat level, cART-combination antiretroviral therapy.
Table 3: Adjusted odd ratio for OSA risk stratified by gender.
The area under the curve (AUC) of the anthropometric indices; BMI, WC, BF%, VFL and WHtR was good with VFL predicting the risk for OSA most accurately (AUC of 0.704, 95% CI 0.651, 0.754), followed by WC (0.668 95% CI 0.613, 0.7I9), WHtR (0.663 95% CI 0.608, 0.715), BMI (0.653 95% CI 0.597, 0.705), and BF% (0.639 95% CI 0.584, 0.692) (Figure 1) (Table 4).
| Variable | AOC | Cut off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Overall accuracy |
|---|---|---|---|---|---|---|---|
| STOP AG | |||||||
| WC (cm) | 0.668 | 80.0 | 84.6 | 45.3 | 17.8 | 95.5 | 51.51 |
| VFL | 0.704 | 7.00 | 51.3 | 82.4 | 29.0 | 92.3 | 77.48 |
| WHtR | 0.663 | 0.50 | 82.1 | 46.4 | 17.6 | 94.8 | 52.38 |
| STOP AG WITH EDS | |||||||
| WC (cm) | 0.706 | 80.0 | 94.4 | 43.8 | 9.2 | 99.2 | 46.84 |
| VFL | 0.770 | 5.00 | 88.9 | 58.5 | 11.4 | 98.9 | 60.32 |
| WHtR | 0.678 | 0.49 | 94.4 | 40.8 | 8.7 | 92.2 | 44.02 |
| STOP AG WITH SSD | |||||||
| WC (cm) | 0.700 | 94.0 | 50 | 83.8 | 17.2 | 96.1 | 81.27 |
| VFL | 0.695 | 5.00 | 75 | 57.9 | 10.7 | 97.2 | 59.18 |
| WHtR | 0.682 | 0.49 | 95 | 40.7 | 9.7 | 99.2 | 44.77 |
Stop ag is with BMI and neck circumference excluded from STOP BANG. WC: waist circumference, WHtR: waist to height ratio, VFL: visceral fat level, EDS: excessive day time sleepiness, SSD: short sleep duration.
Table 4: Cut off values for WC, WHtR and VLF in females for predicting OSA risk.
When we assessed the area under the ROC curves with paired comparisons for VFL, WC, WHtR, BMI and BF%, there were statistically significant differences between VFL and BMI; distance between area is 0.0516 and p value is 0.0081 and also between VFL and BF%; distance between the area is 0.0648 and p value is 0.0075.
We also assessed the AUC for composite score of risk of OSA and EDS (Figure 2), and the AUROC for each metric were also similar when STOPAG was used as the predictor variable and VFL which still had the best predictive ability. In the ROC analysis that was used to determine the optimal cut-off values. We report VFL, WC and WHtR since they have the highest predictive value for predicting the risk of OSA. The values of 80.0 cm for WC (sensitivity, 84.6%; specificity, 45.3%), 7 for VFL (sensitivity, 51.3%; specificity, 82.4%), and 0.50 for WHtR (sensitivity, 82.1%; specificity, 46.4%) were optimal for females.
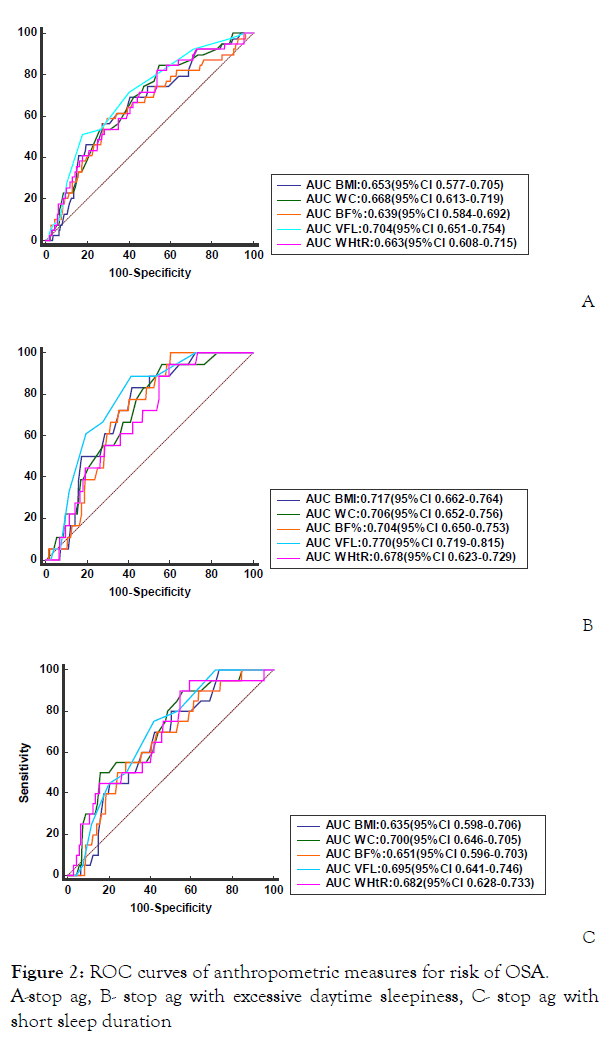
Figure 2: ROC curves of anthropometric measures for risk of OSA.
A-stop ag, B- stop ag with excessive daytime sleepiness, C- stop ag with short sleep duration
In this study, we showed that a moderate proportion (15.8%) of PLHIV presenting for HIV care in a tertiary hospital in Nigeria were at increased risk of OSA. The predictors of OSA risk in females were WC, WHtR and VFL (which are measures of abdominal obesity). Among these, VFL had the highest predictive ability.
Comparing our study to prior studies in PLHIV is challenging as previous data on OSA risk among PLHIV is sparse with varying study designs and methods of assessing OSA risk and diagnosis. Njoh et al. [23] in Cameroon used a case control design and Berlin questionnaire to assess the risk of OSA and documented that PLHIV had higher likelihood of OSA (43.6%) compared to controls (14%) with adjusted OR of 3.9(95% CI: 1.12-13.80) after controlling for socio economic status, depression and smoking. Gutierrez et al. [30] assessed the risk of OSA in a racially and ethnically diverse cohort of PLHIV using the STOP-BANG questionnaire, and found that 58% had intermediate to high risk of OSA. The probable reason for this lower value in this study may be the different populations studied, and we also excluded NC and BMI, even though including these parameters merely increases the percentage to 18.5%.
Conversely, using PSG, in a longitudinal study Chen et.al documented that incidence rate ratio of OSA was lower in the HIV group than in the matched controls (1.91 vs. 2.26 per 1,000 person-years, respectively) in the Taiwanese population [31]. In addition, Kunisaki and colleagues, using electronic and self -reported data documented that 3.99% of HIV infected patients had a diagnosis of OSA as compared to 12.4% in the HIV uninfected population [32]. Patilet al. also documented that OSA was highly prevalent irrespective of HIV status: 86.7% for HIV negative; 70.7%, for HIV-positive on treatment and 73.2% for HIV positive without treatment, despite lower BMI in HIV-positive groups [22]. Considering these latter studies, which is consonance with our own; it does appear that PLHIV may not be at increased risk compared to the general population. The reasons for these findings remain unclear. Future studies are needed to elucidate whether HIV positive patients truly have increased risk of OSA.
In this study, we found that WC, WHtR, BMI, BF%, and VFL were associated with OSA risk after adjusting for demographics and immunological factors in females while HC and NC were not associated with OSA risk. The reason for these findings may not be apparent from this study. It is however plausible that neck fat distribution does not mediate the risk of OSA in PLHIV. The risk of OSA may be mediated by other pathogenetic pathways such as upper airway muscle responsiveness, leptin resistance and decreased arousability from sleep. Examining these other factors may not be practical in routine clinical setting. However more studies will be required among PLHIV to elucidate other pathogenetic mechanism.
Of particular interest, we found that measures of abdominal adiposity namely WC, WHtR and particularly VFL consistently predicted the risk of OSA in females and even after restricting our analysis to those that have concomitant risk of OSA and excessive day time sleepiness. It is well known that heterogeneity of fat deposition may predispose to OSA in the general population. For instance, Vgonstzas et al. revealed that sleep apnea patients had a significantly greater amount of visceral fat compared to obese controls, and indexes of sleep disordered breathing were positively correlated with visceral fat, but not with BMI or total subcutaneous fat [33]. Similarly, Liu et al. demonstrated that mesenteric fat thickness (a component of visceral fat) as measured by ultrasound, had a positive association with presence of moderate OSA and severe OSA, with odds ratio of 7.18 and 7.45 for every 1cm increase in mesenteric fat thickness respectively [34].
Our findings have some clinical implications in terms of management of OSA. Visceral adiposity is an important component of metabolic syndrome (MS) and there is significant evidence of the correlation between MS and OSA [35]. The association between them is complex, not fully elucidated and can be bidirectional. OSA causes sleep fragmentation that can lead to sleep deprivation, daytime somnolence, reduced physical activity, and eventually, weight gain. It is also plausible that insulin resistance, which has been linked with OSA, may lead to weight gain. On the other hand, visceral fat accumulation causes higher leptin production with resistance to said hormone and leading to increase probability of developing OSA [35]. Evaluating VFL in PLHIV would promote screening for other metabolic diseases such as diabetes mellitus, dyslipidemia, and prevent other cardio metabolic diseases as well as early death
Our findings should be interpreted with some caution in view of some limitations. First, we relied exclusively on the use of screening questionnaire to determine the risk of OSA. OSA screening questionnaires tend to have high sensitivity but poor specificity, thereby increasing the number of false-positive results. However, we also evaluate the composite risk of OSA and EDS. EDS is an important symptom of OSA often prompting patients to seek medical attention Therefore, incorporating the symptoms of sleepiness would reduce the occurrence of false positivity and increase the probability of OSA diagnosis. Second, we measured VFL by BIA. BIA may overestimate body fat composition in comparison to computed tomography, magnetic resonance imaging, or dual energy X-ray absorptiometry; however, it is a comparatively cheap technique for estimating body composition. it emits no radiation, requires little or no training and only few minutes of the participant’s time is required. In conclusion, the risk of OSA among PLHIV is associated with measures of visceral adiposity in females and not in males. Sleep is essential and needed for several neurobiological processes including immune system modulation. Using OSA screening questionnaires and simple anthropometric measurements can expedite the diagnosis and management of OSA and other metabolic diseases. This may prevent related complication of both HIV and OSA, and greatly improve the quality of life PLHIV.
We wish to express our sincere gratitude to all PLHIV who participated in the study for their co-operation. Our heartfelt thanks to nursing staff HIV unit of Obafemi Awolowo University Teaching Hospitals Complex, Ile Ife, Osun state, Nigeria.
The authors report no conflict of interest and this research was not supported by any organization.
Citation: Awopeju OF, Oninla OA, Odeyemi AO, Adebowale AA, Awoniyi FO, Zifodya JS, et al. (2020) Gender-Specific Association of Body Fat Composition with Risk of Obstructive Sleep Apnea among People Living With HIV/AIDS: A Cross-Sectional Study. J Sleep Disord Ther 9:324.
Received: 30-Sep-2020 Accepted: 16-Nov-2020 Published: 23-Nov-2020 , DOI: 10.35248/2167-0277.20.9.324
Copyright: © 2020 Awopeju OF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.