Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Review Article - (2021)
Endotoxin and its induced exaggerative responses of proinflammatory cytokines such as Tumor Necrosis Factor (TNF) cause human septic shock. The generalized Shwartzman reaction is considered as an experimental endotoxin shock model and its representative features are high lethality and hypersensitive inflammatory response to endotoxin, in particular excessive TNF production. The generalized Shwartzman reaction can be induced by Interleukin (IL)-12 priming and subsequent endotoxin challenge. IL-12 primes the intermediate T cell receptor (TCR)-expressing innate T lymphocytes (natural killer T cells and CD8+CD122+ T cells in mice, CD56+ T cells and CD57+ T cells in human) to produce IFN-γ, which in turn preconditions macrophages to produce a large amount of TNF in response to endotoxin. These intermediate TCR innate T lymphocytes play a pivotal role for the occurrence of this generalized Shwartzman reaction. Some of the intermediate TCR-expressing innate T lymphocytes (CD8+CD122+ T cells in mice and CD57+ T cells in human), which are considered as extrathymically developed T lymphocytes, are increased with age and closely involved in age-dependent augmentation of murine generalized Shwartzman reaction and human Shwartzman-like reaction, which may reflect an exacerbation of septic shock in elderly. However, these intermediate TCR innate T lymphocytes normally augment host immunity against malignant tumors and refractory bacterial infections, which are critical threats in aged hosts. Thus, intermediate TCR-expressing innate T lymphocytes may be a double-edged sword on the host defense, especially elderly hosts.
Elderly septic shock; Extrathymicall T lymphocytes; Natural killer T cells; CD8+CD122+ T cells; CD56+ T cells; CD57+ T cells
Septic shock especially caused by gram-negative bacterial infection still has shown a persistent high mortality, although many researchers vigorously investigated effective therapies for it [1,2]. Aging is an obvious risk factor of septic shock and the mortality from septic shock in elderly is quite high [3-5]. Endotoxin, gram-negative bacterial toxin, is well-known to cause septic shock [6,7]. Severe sepsis/septic shock have been considered to be a result of overly exuberant inflammatory responses of the hosts, which are evoked by exaggerated proinflammatory cytokine secretions [4]. Generalized Shwartzman reaction also induces hypersensitive proinflammatory cytokine response to endotoxin, which is the reason why the generalized Shwartzman reaction is thought to be an experimental endotoxin shock model [8,9]. We have studied the intermediate T cell Receptor (TCR)-expressing T lymphocytes that are involved in the innate immunity not acquired immunity [10]. Interestingly, these innate T lymphocytes are closely involved in the pathogenesis of generalized Shwartzman reaction. In this review, we demonstrated the mechanisms of generalized Shwartzman reaction, focusing on these innate T lymphocytes, and also addressed age-dependent augmentation of the generalized Shwartzman reaction, which is one of the key phenomena to resolve why the elderly susceptible to septic shock.
Original local Shwartzman reaction and the generalized Shwartzman reaction
Shwartzman phenomenon was originally reported that the intradermal injection of the sterile culture filtrate of the Gram- negative bacterium Bacillus Salmonella Typhosus as a preparatory injection and the subsequent intravenous injection with a provocative dose of the same culture filtrate 24 h later induced severe hemorrhagic necrosis at the first injection site in rabbits [11]. Shwartzman only described the localized dermal response but not the systemic response in this phenomenon.
However, importantly he also described that this dermal necrosis was not induced by the sterile culture filtrate of Gram-positive streptococcal species, suggesting that the heat-stable component of Gram-negative bacteria, later identified as endotoxin, caused this phenomenon [8].
According to the review of Chahin et al., Sanarelli reported more generalized pathological findings (multiple organ dysfunctions) in rabbits given a sensitizing intravenously, followed by a second provocative intravenous dose of culture filtrates from Vibrio cholerae [8]. Notably, it was four years earlier than the first report by Shwartzman.
Criteria of the generalized Shwartzman reaction; high lethality and hypersensitive TNF response to endotoxin
High lethality as well as hypersensitive inflammatory response to endotoxin are the most representative characteristics of generalized Shwartzman reaction [3,9]. Such hypersensitive conditions are induced by a preceding priming stimulation with low dose endotoxin (Figure 1A). Endotoxin, also called Lipopolysaccharide (LPS), is a gram-negative bacterial cell-wall component [7].
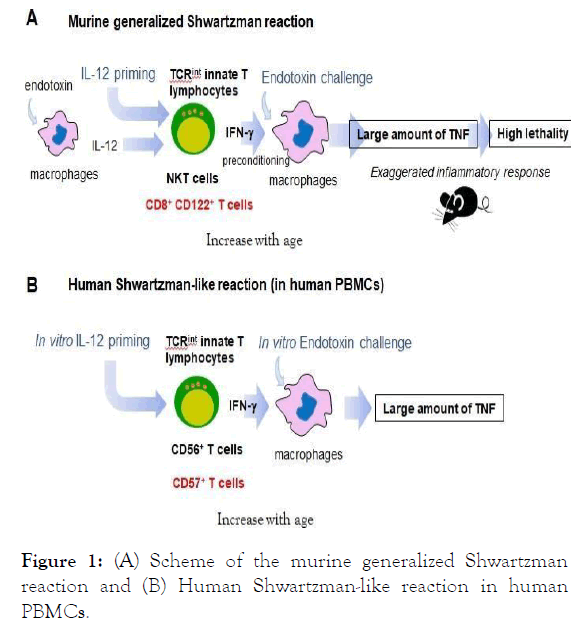
Figure 1: (A) Scheme of the murine generalized Shwartzman reaction and (B) Human Shwartzman-like reaction in human PBMCs.
Endotoxin/LPS bind to the Toll-Like Receptor 4 (TLR4) on the mammalian cell membrane and stimulate the inflammatory signal cascade to produce proinflammatory cytokines, such as Tumor Necrosis Factor (TNF) [12,13].
Macrophages substantially express TLR4 on their cell membranes and potently produce TNF by the stimulation of endotoxin. In human, administration of endotoxin to healthy volunteers caused sepsis-like symptoms and increased the plasma TNF levels [14,15]. In turn, administration of TNF to volunteers evoked similar inflammatory reaction to that of endotoxin challenge [14,16].
Taken together, TNF presumably plays a pivotal role for human endotoxemia/Gram-negative sepsis. Therefore, we think that hypersensitive TNF production by activated/primed macrophages in response to endotoxin can be said as one of the important criteria of generalized Shwartzman reaction (Figure 1A). Of course, its induced high lethality is also a representative feature of generalized Shwartzman reaction (Figure 1A).
Interleukin (IL)-12-primed generalized Shwartzman reaction
Priming of macrophages by preceding stimulation with endotoxin or its derivatives is indispensable to induce these hypersensitive condition and high lethality in the generalized Shwartzman reaction. However, repeated stimulation with endotoxin may induce a kind of endotoxin tolerance in the hosts, which can block some of the hypersensitivity features shown by the generalized Shwartzman reaction [17,18].
Actually, when we attempted to induce generalized Shwartzman reaction in mice by endotoxin-priming and subsequent endotoxin-challenge, no mice died [19]. In contrast, when we primed mice with intraperitoneal injection of IL-12 instead of endotoxin, this IL-12 priming certainly enabled to induce hypersensitive response to the following endotoxin challenge with high mortality (Figure 1A).
Intermediate TCR-expressing innate T lymphocytes in mice and humans
Liver is a crucial immune organ for the host defense [10], and we have studied the immune cells in the liver. Several decades ago, we found a unique subset in the liver lymphocytes, showing intermediate αβTCR intensity (Figure 2), and upper panel [20].
We could find such obvious intermediate TCR-expressing population in neither spleen nor thymus (Figure 2), middle and lower panel [10]. Intensity of this intermediate αβTCR is higher than that of thymic immature CD4+ CD8+ double-positive cells, which is called dull TCR, but lower than that of regular mature T lymphocytes (high TCR) (Figure 2).
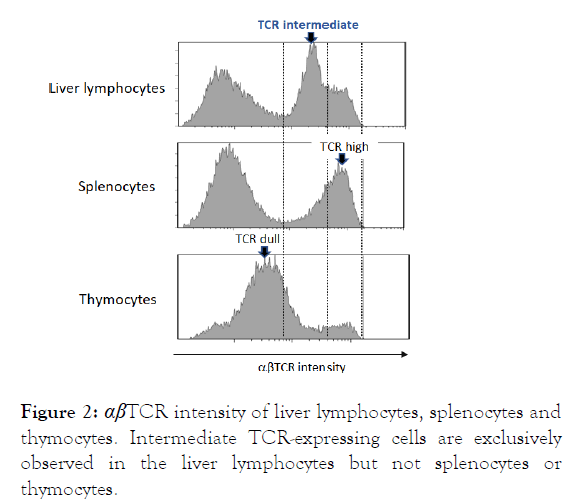
Figure 2: αβTCR intensity of liver lymphocytes, splenocytes and thymocytes. Intermediate TCR-expressing cells are exclusively observed in the liver lymphocytes but not splenocytes or thymocytes.
In addition, this intermediate αβTCR is distinct from the regular αβTCR, because it shows lower TCR diversity (repertoire) than that of the regular TCR [10,21,22], thereby these T lymphocytes are exclusively involved in the innate immunity not acquired immunity leading to produce antigen- specific antibodies. These intermediate TCR expressing cells mainly exist in the liver and interestingly some of them are considered as extrathymically developed T lymphocytes [23,24]. NK1.1 is a murine marker of Natural Killer (NK) cells corresponding to human CD161 [25]. Approximately two thirds of intermediate TCR cells in the liver are NK1.1+ (also CD122+) and one third are NK1.1- but CD122+ [22,10]. The former are called Natural Killer T (NKT) cells and mainly CD4+, while the latter are mainly CD8+, thereby called CD8+CD122+ T cells. In human, CD56+ T cells (most of them express CD161) and CD57+ T cells also express an intermediate TCR intensity, thereby considering as a counterpart of murine NKT cells or CD8+CD122+ T cells [10,25].
Intermediate TCR-expressing innate T lymphocytes are crucial for the generalized Shwartzman reaction
IL-12, one of the representative Th1 cytokines, is mainly produced from macrophages and potently stimulates NK, NKT, and T cells to produce IFN-γ [26,27]. When the mice are primed with endotoxin, macrophages produce IL-12 and stimulate NK, NKT and T cells to produce IFN-γ. In turn, this IFN-γ preconditions macrophages, which process is important for macrophages to hypersensitive response to endotoxin (Figure 1A) [28]. Interestingly, by the current murine generalized Shwartzman reaction, IL-12 priming stimulates NKT cells but not NK or T cells, because deletion of NK cells alone in mice could not inhibit the generalized Shwartzman reaction but the additional deletion of NKT cells drastically reduced the lethality of generalized Shwartzman reaction accompanied with potent reduction of IFN-γ in the priming phase [19,28]. Consistently, IL-12 also can enhance murine antitumor cytotoxicity via activation of NKT cells [29]. Nevertheless, exaggerated TNF secretion from macrophages by the subsequent endotoxin challenge was not reduced by the deletion of NKT/NK cells [19,28]. In the priming phase, IL-12 also potently activates murine CD8+CD122+ T cells as well as NKT cells to produce IFN-γ (Figure 1A) [28]. Interestingly, deletion of CD8+CD122+ T cells in addition to NKT/NK cell deletion in mice significantly reduced the plasma TNF levels by the subsequent endotoxin challenge, accompanied with potent reduction of mortality and IFN-γ production after IL-12 priming. Both NKT cells and CD8+CD122+ T cells that are innate T lymphocytes showing intermediate TCR expression thus play crucial roles for the occurrence of generalized Shwartzman reaction.
CD8+ CD122+ T cells that increase with age are involved in the age-dependent enhancement of generalized Shwartzman reaction
Age-associated thymus atrophy is well-known [30]. Unlike thymus-derived regular T lymphocytes, CD8+CD122+ T cells, which are considered as extrathymically developed T lymphocytes [23,24], increase with age in mice [10,31]. Murine NKT cells are also intermediate TCR-expressing cells but do not show such an age-dependent increase [31]. Interestingly, murine generalized Shwartzman reaction drastically enhances its lethality and TNF response with aging [28].
Age-dependently increasing CD8+CD122+ T cells are closely involved in this age- associated enhancement of generalized Shwartzman reaction in mice, because deletion of CD8+CD122+ T cells abrogated these age-dependent phenomena while adoptive transfer of CD8+CD122+ T cells to young mice augmented the generalized Shwartzman reaction like aged mice [28].
Human Shwartzman-like reaction show an age- dependent augmentation
About five decades ago, Starzl reported three patient cases of renal transplantation who were suspected of a Shwartzman reaction [32]. However, we ethically cannot perform a human trial of the generalized Shwartzman reaction. We then examined in vitro Shwartzman-like reaction using human Peripheral Blood Mononuclear Cells (PBMCs) [3]. We primed the human PBMCs with IL-12 and 24 h later challenged them with endotoxin (Figure 1B). IL-12 priming stimulated CD56+ T and CD57+ T cells in the PBMCs to produce IFN-γ that in turn activated/preconditioned macrophages in the PBMCs to up- regulate TLR4 expression (Figure 1B). Thereafter, these macrophages produced large amount of TNF by in vitro endotoxin challenge (Figure 1B), suggesting that hypersensitive TNF production in response to endotoxin was also induced in human lymphocytes/macrophages like murine generalized Shwartzman reaction [3]. Interestingly, PBMCs from the elderly healthy volunteers enhanced this Shwartzman-like reaction while PBMC from the child volunteers reduced this reaction [3].
We confirmed the age-dependent increase in CD57+ T cells in that study. In humans, generalized Shwartzman reaction may possibly occur, and its age-dependent augmentation also can be induced, although the differences in the host immune system certainly exist between humans and mice.
Elderly patients are susceptible to septic shock with marked elevation of plasma TNF levels
In our previous study, elderly patients with intraabdominal infection (over 70 years old) showed higher plasma TNF levels than that of adult patients (less than 70 years old) (elderly 19 pg/mL vs. adult 10 pg/mL in average, p<0.05), although both patient groups showed similar plasma endotoxin levels (elderly
7.4 pg/mL vs. adult 7.6 pg/mL) (Figure 3). Notably, these elderly patients showed remarkably higher septic shock rates and its mortality than those of adult patients (shock rate, 61% vs. 20%, p<0.01; mortality, 91% vs. 25%, p<0.05) (Figure 3) [3].
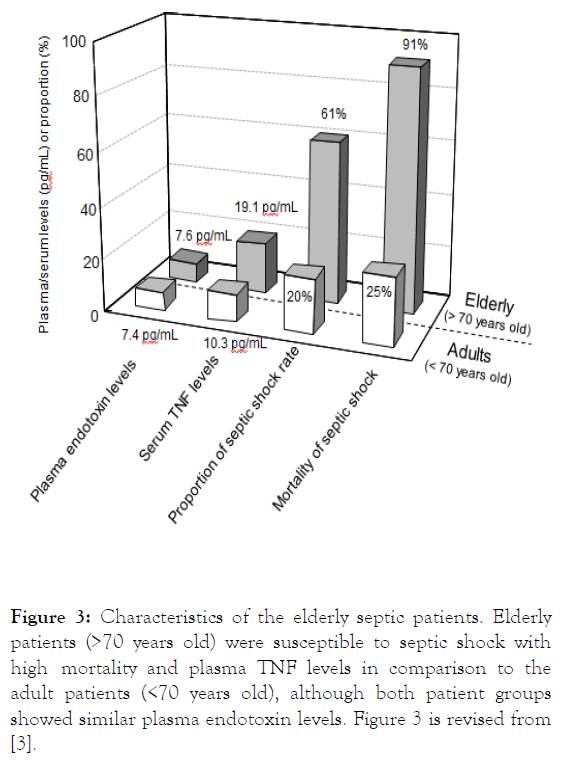
Figure 3: Characteristics of the elderly septic patients. Elderly patients (>70 years old) were susceptible to septic shock with high mortality and plasma TNF levels in comparison to the adult patients (<70 years old), although both patient groups showed similar plasma endotoxin levels. Figure 3 is revised from [3].
These patients suffered from the lower gastrointestinal perforation, anastomotic breakdown after gastrointestinal surgery, or Acute Obstructive Suppurative Cholangitis (ASOC). There are possibilities of some preceding infectious stimulation followed by the Gram-negative bacterial insults; thereby a kind of age-dependently enhanced generalized Shwartzman reaction may occur. Acute Respiratory Distress Syndrome (ARDS) in COVID-19 patients showed hyper inflammatory cytokine response [33] and elderly appear to be susceptible to this COVID-19 ARDS [34]. Although it is a new type of coronavirus infection, not bacterial sepsis, innate T lymphocytes and infiltrating monocytes/macrophages, especially in the lung, may possibly be involved in the age-related exacerbation of this COVID-19 ARDS.
Why are intermediate TCR-expressing innate T lymphocytes increased with age? Their beneficial roles for the host defense
Finally, some questions are raised why the intermediate TCR- expressing innate T lymphocytes increase with age. To address this issue, we induced the CD8+CD122+ T cells in young mice by multiple IL-15 treatment (alternative day challenge for one week) [35]. IL-15 is a growth factor that used the β and γ chains of the IL-2 receptor for signal transduction in conjunction with the α-chain of the IL-15-specific receptor [36,37]. CD122 is an IL-2 receptor β-chain that is a functional receptor for IL-15 and controls the proliferation and survival of CD8+CD122+ T cells [38]. Increase in the CD8+CD122+ T cells rendered young mice susceptible to the generalized Shwartzman reaction like aged mice. However, notably these mice augmented not only antitumor cytotoxicities but also bacterial elimination activity in comparison to the non-treated young mice [35]. The mammalian hosts may obtain these intermediate TCR- expressing innate T lymphocytes that are increased with age, in order to augment host defense immunity against malignant tumors and refractory bacterial infections, which are critical threats in the aged hosts. However, such immune systems involved in the intermediate TCR-expressing innate T lymphocytes may be a double-edged sword, because they also enhance the generalized Shwartzman reaction.
We think that the representative features of generalized Shwartzman reaction are high lethality and hypersensitive TNF response to endotoxin. The intermediate TCR-expressing innate T lymphocytes including extrathymically developed T lymphocytes are crucial for the occurrence of this generalized Shwartzman reaction. Some of these intermediate TCR- expressing innate T lymphocytes, CD8+CD122+ T cells in mice and CD57+ (CD122+) T cells in humans increase with age and are closely involved in the age-dependent augmentation of generalized Shwartzman reaction, although they also augment antitumor immunity and antibacterial immunity, which are critical for the aged hosts.
Citation: Kinoshita M, Nakashima M, Seki S, Nakashima H (2021) Generalized Shwartzman Reaction as an Experimental Endotoxin Shock Model: Role of Intermediate T Cell Receptor-Expressing Innate T Lymphocytes in its Pathogenesis. Fungal Genom Biol. S2:001.
Received: 30-Mar-2021 Accepted: 13-Apr-2021 Published: 20-Apr-2021 , DOI: 10.35248/2165-8056.21.s2.001
Copyright: © 2021 Kinoshita M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.