Research Article - (2017) Volume 3, Issue 1
Genetic Characterization of Rhizosphere Bacteria that Inhabit Common Bean Nodules in Western Kenya Soils
*Corresponding Author: Clabe Wekesa, Department of Biological Sciences, Masinde Muliro University of Science and Technology, Kenya Email:
Abstract
Background: With the increasing world population, there is increasing demand for food. This has led to overuse of agricultural farms causing reduced soil fertility and accumulation of phytopathogens. Inorganic fertilizers and pesticides have been extensively used in response to these challenges. Extensive integration of inorganic fertilizers and pesticides in the farming system has contributed to soil and water pollution worsening the eutrophication in rivers lake waters. Alternative farming methods are therefore necessary to address this problem. Recent studies have found that rhizobacteria that colonize nodules of leguminous plants are capable of increasing yield and health of the tested plants. Their plant growth promoting ability depends on the rhizobacteria type, soil properties, and climatic conditions. The aim of this study, therefore, was to genetically characterize rhizobacteria that closely associate with common bean nodules by analyzing the nucleotide sequence of 16SrRNA gene. Results: The 16SrRNA gene analysis revealed that common bean nodule associated bacteria in Western Kenya soils are genetically diverse as indicated by the evolutionary genetic distances. Not even organisms in the same species had zero genetic distance though they formed independent groups on the phylogenetic tree. The isolates belonged to the genus Pseudomonas, Providencia, Rhizobia, Klebsiella, Enterobacter, Delfitia and Acinetobacter as identified through nucleotide BLAST at the NCBI GenBank database. Conclusion: Rhizobacteria that colonize common bean nodules are genetically diverse. Those found in this study may be adaptable to Western Kenya soils and further tests are required to determine their plant growth promoting efficiency.
Keywords: Rhizobacteria; Nodule associated bacteria; Nitrogen fixation; Phylogenetics
Background
A large proportion of the population in Western Kenya is involved in agricultural production and the common bean is one of the major crop grown [1]. Due to a rapid increase in population growth, there is a high demand for food production, hence farms are repeatedly used. This habit has greatly reduced soil fertility and bred more phytopathogens resulting to very low yield [2]. In order to increase the crops yields, farmers have therefore resorted to the intensive use of inorganic fertilizers in an attempt to boost fertility in their farms and use of pesticides to reduce damage by phytopathogens. Inorganic fertilizers and pesticides may increase the accumulation of heavy metals in the soil and plant systems [3]. Inorganic fertilizers mainly contain ammonia, phosphates, potassium and nitrate salts. These salts reach the water bodies through leaching, drainage, and flow. Water pollution by these inorganics constitutes a major concern globally as it may lead to the onset of many fatal diseases in humans, direct killing of aquatic animals, eutrophication in water bodies and bioaccumulation of these toxic compounds in food trophic levels [4].
The economic consequences of the application of inorganic fertilizers together with their negative impacts on the environment have become a concern globally thus, there is a need for farmers to shift to the farming practices that are sustainable [5]. Studies have shown that the use of plant growth promotion rhizobacteria (PGPR) can significantly increase the yield of common bean [6-9] and hence it is a potential alternative to heavy dependency on inorganic fertilization and use of pesticides. The mechanisms by which these soil microorganisms promote plant growth are not well elucidated but nitrogen fixation [10-12], phosphorus solubilization [13,14] and inhibition of phytopathogens growth [15,16] are thought to be a possible explanation for this effect. The belief that only rhizobia colonize nodules of leguminous plants is disputable. Researchers have been isolating other microorganisms besides rhizobia as bona fide members of nodules in the legumes [6,17]. They have demonstrated the isolation of bacteria of several genera; Pseudomonas, Aerobacter, Agrobacterium, Chryseomonas, Bacillus, Curtobacterium, Erwinia, Enterobacter, Sphingomonas , and Flavimonas . The presence of these bacteria in the nodules is not accidental. Available reports over time have shown that co-inoculation of rhizobia with other rhizobacteria tremendously increased the yield of common beans compared to when rhizobia were the only inoculant in terms of increased number of pods per plant, the number of seeds per pod, weight of pods per plant and total dry matter of the shoot [18]. Rajendran et al. [19] reported an increased nodulation and root weight in greenhouse conditions when common beans were co-inoculated with rhizobia together with other nodule associated bacteria. Nodule associated bacteria that so far have been co-inoculated with rhizobia include Azospirillum [20], Azotobacter , Bacillus [21] and Pseudomonas [13]. All these experiments resulted in increased yields due to improved nutrient availability and plant health.
Various studies have found that plant growth-promoting rhizobacteria (PGPR) strains vary widely in different soils and their ability to promote growth may be highly specific to particular species, cultivar, soil and genotype [17]. Under such circumstances, knowledge of native bacterial population and their identification is important for understanding their distribution and diversity [22]. It is important to explore and identify region-specific microbial strains which can be used as potential plant growth promoters to achieve higher yields under specific ecological and environmental conditions. There are no published studies that characterized the rhizobacteria in the soils of Western Kenya associated with nodules of Phaseolus vulgaris and therefore efforts to establish inoculants that are specific for these soils have been elusive. Information concerning the genetic diversity and distribution of these important microbes is thus necessary for the production of PGPR inoculants specific for this region.
Molecular techniques have successfully been used in examining microbial identity and diversity [23]. Mostly these studies have utilized sequence analysis of 16SrRNA gene which is highly conserved in all prokaryotes [24,25]. The conservation of this gene has enabled synthesis of primers that target various taxonomic groups but have enough variations to give phylogenetic comparisons of different microbial communities [26,27].
The composition of microbial communities can be analyzed based on profiles generated from physical separation of 16SrRNA gene sequences on the gel [23]. These techniques detect different sizes of PCR-amplified 16SrRNA gene fragments. Direct sequencing of 16SrRNA gene has been employed in establishing genetic relationships and characterization of strains at the generic or higher level [28]. Sequencing techniques have increased tremendously due to the invention of next-generation sequencing that has reduced the cost of sequencing [29] making the technique affordable even to low income researchers.
Methods
Study site
Nodules of common beans were collected from farmers’ fields in which there is no history of inoculation with any nodule associated bacteria but in which common bean has been grown frequently. Nodules were collected from the slopes of Mt. Elgon, shores of Lake Victoria at Kisumu and Kakamega. At the shores Lake Victoria, nodules were collected from farm A (S 00° 08.729’; E 034° 69.596’), Farm B (S 00° 08.828’; E 034° 69.654’), Farm C (S 00° 08.852’; 034° 69.654’) and Farm D (S 00° 09.094; E 034° 69.715’), all in Korando sublocation in Kisumu County. At Mt. Elgon region, soils were collected from Farm A (S 00° 79.209’; E 034° 63.688’), Farm B (S 00° 77.913’; E 034° 64.030’), Farm C (S 00° 81.852’; 034° 61.654’) and Farm D (S 00° 82.094; E 034° 59.715’), all in Kapkateny sub-location in Bungoma County. At Kakamega, soils and nodules were collected from Farm A (S 00° 19.570’; E 034° 65.921’), Farm B (S 00° 20.779’; E 034° 65.663’), Farm C (S 00° 18.982’; 034° 68.534’) and Farm D (S 00° 18.715; E 034° 68.607’), all in Kakamega South sub-county in Kakamega County. Collection strategy employed the randomized technique in which nodules were collected six meters apart following a W pattern running across the whole plot. Three common bean plants were collected from each site. The uprooted plants were packed in khaki bags and transported to the Microbiology laboratory at Masinde Muliro University of Science and Technology, Kenya for analysis.
Isolation of nodule associated bacteria
Nodule associated bacteria were isolated from surface-sterilized nodules according to the method described by Rincon et al. [30]. The nodule surfaces were first sterilized with 75% ethanol, followed by 0.1% mercuric chloride for about 3 min and then extensively rinsed six times with sterile distilled water. The water from the sixth rinse was streaked on yeast extract mannitol agar (YMA) to confirm the complete removal of nodule epiphytes before the nodules were crushed with a flame-sterilized blunt-tipped pair of forceps. The exudates of the crushed nodules were cultured on yeast-mannitol agar (YMA) medium at 28°C for 3 days, and a single colony was selected for further culture. The validation of the culture purity was performed by repeated streaking on Yeast extract mannitol agar medium and cellular examination in the microscope. The isolates were then stored in 20% glycerol at -70°C.
DNA extraction, PCR amplification, and sequencing of 16SrRNA gene
Genomic DNA was isolated using QIAamp® genomic DNA kit following the manufacturer’s instructions and 16SrRNA gene was amplified using the universal primers, 27f (5’AGAGTTTGATCCTGGCTCAG 3’) and 1492r (5' TACGGCTACCTTGTTACGACTT 3') which are complementary to conserved regions of the bacterial 16SrRNA gene. Amplification was carried out in 25 μL reaction volumes containing the following: 2.5 μL 10X PCR reaction buffer (100 mM Tris-HCl, pH 8.3, 500 mM KCl) and 1.5 μL 25 mM MgCl2 solution, 4.0 μL 1.25 mM, dNTPs, 0.5 μL of 27f primer (200 ng/μL), 0.5 μL of 1492r primer (200 ng/μl), 0.1 μL AmpliTaq Gold DNA polymerase and 1 μL of DNA as template. The reaction volume was adjusted up to 25 μL with sterile ultrapure water. The PCR thermal cycling conditions consisted of an initial denaturation step at 94°C for 3 min, followed by 30 cycles of denaturation (1 min at 94°C), annealing for 1 min at 57°C and extension for 2 min at 72°C, followed by a final extension at 72°C for 8 min. Double distilled water was used as negative control to check for false positive as a result of contamination of the reagents. PCR amplified products were separated on 1.0% agarose gels in 1X TBE buffer at 10 V cm-1 for 30 minutes.
After the gel was photographed, the bands were located by using UV lamp, cut out and placed in a 2 mL Eppendorf tube. The PCR fragments were then extracted from the gel using Qiagen Gel purification kit following the manufacturer’s instruction. Sequencing reactions were performed at Bioneer, South Korea using the BigDye Terminator v3.1 sequencing Kit (Applied Biosystems, USA) with the primers 27f, and 1492r and sequenced products were analyzed using an automatic sequencer, ABI3730XL (Applied Biosystems).
Phylogenetic data analysis
Consensus sequences of the forward and reverse primers were generated in BioEdit ver. 7 [31] and then nucleotide alignment was generated by CLUSTAL W [32] implemented in BioEdit ver. 7. The alignment file was then loaded in MEGA 6 where the evolutionary history was inferred using the Neighbor-Joining method [33]. The bootstrap consensus tree inferred from 1000 replicates [34] was taken to represent the evolutionary history of the taxa analyzed [34]. Branches corresponding to partitions reproduced in less than 50 % bootstrap replicates were collapsed. The evolutionary distances were computed using the Jukes-Cantor method [35] and are in the units of the number of base substitutions per site. The analysis involved 24 nucleotide sequences. Codon positions included were 1st, 2nd, 3rd and noncoding. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA5 [36].
Results
Genetic diversity and distribution of nodule associated bacteria
This study reports a total of 24 strains of rhizobacteria isolated from common bean nodules, including Delfitia , Rhizobia , Acinetobacter , Pseudomonas , Providencia , Enterobacter , and Klebsiella . The 24 sequences submitted to the NCBI GenBank were assigned accession numbers as shown in Table 1.
| Organism name | Strain/sample | NCBI Accession number |
|---|---|---|
| Enterobacterhormaechei | E1 | KX856071.1 |
| Pseudomonas koreensis | E2 | KX856072.1 |
| Providenciarettgeri | E3 | KX856073.1 |
| Providenciarettgeri | E4 | KX856074.1 |
| Pseudomonas koreensis | E5 | KX856075.1 |
| Providenciarettgeri | E6 | KX856076.1 |
| Enterobacter cloacae | E8 | KX856077.1 |
| Pseudomonas sp. | E9 | KX856078.1 |
| Pseudomonas sp. | E10 | KX856079.1 |
| Enterobacter sp. | K1 | KX856080.1 |
| Klebsiellapneumoniae | K2 | KX856081.1 |
| Providencia sp. | K3 | KX856082.1 |
| Pseudomonas koreensis | K5 | KX856083.1 |
| Enterobacterhormaechei | K6 | KX856084.1 |
| Delftia sp. | K7 | KX856085.1 |
| Rhizobium sp. | S2 | KX856086.1 |
| Delftia sp. | S3 | KX856087.1 |
| Rhizobium sp. | S4 | KX856088.1 |
| Delftia sp. | S5 | KX856089.1 |
| Rhizobium sp. | S6 | KX856090.1 |
| Delftialacustris | S7 | KX856091.1 |
| Delftialacustris | S8 | KX856092.1 |
| Enterobacterasburiae | S9 | KX856093.1 |
| Acinetobactercalcoaceticus | S10 | KX856094.1 |
Table 1: NCBI identity of nodule associated bacteria (NAB) obtained from nodules of common beans.
Phylogenetic analysis
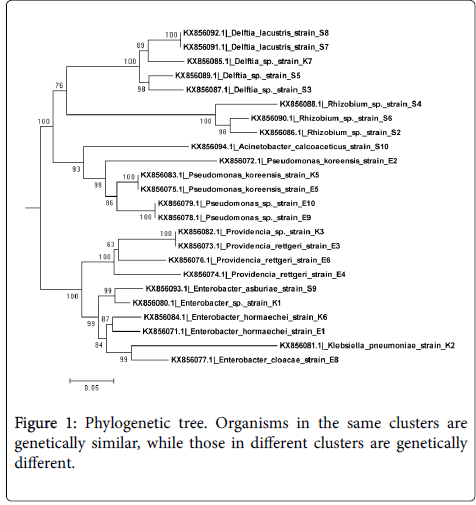
The isolates clustered into five clades on the phylogenetic tree shown in Figure 1, Clade A contained Delfitia spp. With GenBank accession numbers KX856092.1, KX856091.1, KX856085.1, KX856089.1, and KX856067.1. Clade B contained Rhizobia spp ., KX856088.1, KX856090.1, and KX856086.1. Both of these clustered were supported by 100% bootstrap confidence. Clade C contained members of Pseudomonas spp. with accession numbers KX856083.1, KX856075.1, KX856079.1, KX856078.1 and KX856078.1 whose branch was supported by 99% bootstrap confidence. Members of Providencia spp. formed Clade D with a branch supported by 100% bootstrap confidence and they included strains with accession numbers KX856082.1, KX856073.1, KX856076.1, and KX856074.1. Finally, Enterobacter spp. formed Clade E which contained strains with accession numbers KX856093.1, KX856080.1, KX856084.1, KX856071.1, and KX856077.1.
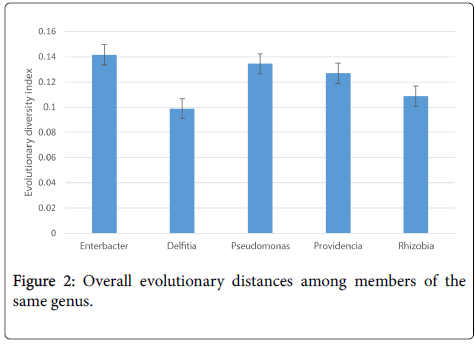
Most of the isolates in each genus were genetically diverse with the exception of only a few members (Table 2). Among the genera Enterobacter , all the species were genetically diverse, the maximum [max] distance was 0.1831 ± 0.0125 between Enterobacter asburiae strain S9 and Enterobacter cloacae strain E8 while the minimum evolutionary distance was 0.0761 ± 0.0077 between Enterobacter hormaechei strain K6 and Enterobacter hormaechei strain E1. The overall evolutionary distance among members of Enterobacter was 0.1416. Among members of Delfitia , the maximum evolutionary distance was 0.1183 ± 0.009 between Delftia sp. strain S3 and Delftia lacustris strain S7 and Delftia lacustris strain S8. Delfitia lacustris strains S7 and S8 had exactly 0.00 evolutionary distances meaning they were genetically identical. The overall evolutionary distance of Delfitia was 0.0988. Maximum evolutionary distance among members of Pseudomonas was 0.2553 ± 0.0152 between Pseudomonas koreensis strain E2 and Pseudomonas sp. strain E9 and Pseudomonas sp. strain E10. Genetic distance between Pseudomonas sp. strains E9 and E19 and Pseudomonas koreensis strain K5 and Pseudomonas koreensis strain E5 was 0.00. The overall mean evolutionary distance among members of Pseudomonas was 0.1344. The minimum evolutionary distance among members of Providencia was 0.1707 ± 0.0129 between Providencia rettgeri strain E4 and Providencia sp. strain K3 and Providencia rettgeri strain E3. Again, Providencia sp. strain K3 and Providencia rettgeri strain E3 had an evolutionary distance of 0.00 meaning that they were genetically identical. The overall genetic distance among members of these genera was 0.1269. In rhizobia genera , Rhizobium_sp. Strain S6 and Rhizobium sp . Strain S4 had the highest evolutionary distance of 0.1375 ± 0.0115. The overall genetic distance among members of Rhizobia was 0.1088. It follows that members of the genera Enterobacter were more diverse and members of Delfitia were less genetically diverse as shown in Figure 2.
| Species 1 | Species 2 | Dist. | Err |
|---|---|---|---|
| KX856093.1|Enterobacter asburiaestrain S9 | KX856084.1|Enterobacter hormaechei strain K6 | 0.1485 | 0.0117 |
| KX856093.1|Enterobacter asburiaestrain S9 | KX856080.1|Enterobacter sp. strain K1 | 0.0916 | 0.0093 |
| KX856084.1|Enterobacter hormaecheistrainK6 | KX856080.1|Enterobacter sp. strain K1 | 0.1373 | 0.0105 |
| KX856093.1|Enterobacter asburiaestrain S9 | KX856077.1|Enterobacter cloacae strain E8 | 0.1831 | 0.0125 |
| KX856084.1|Enterobacter hormaecheistrain K6 | KX856077.1|Enterobacter cloacae strain E8 | 0.1608 | 0.012 |
| KX856080.1|Enterobacter sp.strain K1 | KX856077.1|Enterobacter cloacae strain E8 | 0.1714 | 0.0116 |
| KX856093.1|Enterobacter asburiae strain S9 | KX856071.1|Enterobacter hormaechei strain E1 | 0.1457 | 0.0122 |
| KX856084.1|Enterobacter hormaecheistrain K6 | KX856071.1|Enterobacter hormaechei strain E1 | 0.0761 | 0.0077 |
| KX856080.1|Enterobacter sp. strain K1 | KX856071.1|Enterobacter hormaechei strain E1 | 0.1373 | 0.0107 |
| KX856077.1|Enterobacter cloacae strain E8 | KX856071.1|Enterobacter hormaechei strain E1 | 0.1646 | 0.012 |
| KX856092.1|Delftia lacustris strain S8 | KX856091.1|Delftia lacustris strain S7 | 0 | 0 |
| KX856092.1|Delftia lacustris strain S8 | KX856089.1|Delftia sp. strain S5 | 0.113 | 0.0089 |
| KX856091.1|Delftia lacustris strain S7 | KX856089.1|Delftia sp. strain S5 | 0.113 | 0.0089 |
| KX856092.1|Delftia lacustris strain S8 | KX856087.1|Delftia sp. strain S3 | 0.1183 | 0.009 |
| KX856091.1|Delftia lacustris strain S7 | KX856087.1|Delftia sp. strain S3 | 0.1183 | 0.009 |
| KX856089.1|Delftia sp. strain S5 | KX856087.1|Delftia sp. strain S3 | 0.1034 | 0.0083 |
| KX856092.1|Delftia lacustris strain S8 | KX856085.1|Delftia sp. strain K7 | 0.0965 | 0.0083 |
| KX856091.1| Delftialacustris strain S7 | KX856085.1|Delftia sp. strain K7 | 0.0965 | 0.0083 |
| KX856089.1|Delftia sp. strain S5 | KX856085.1|Delftia sp. strain K7 | 0.1121 | 0.009 |
| KX856087.1|Delftia sp. strain S3 | KX856085.1|Delftia sp. StrainK7 | 0.1165 | 0.0088 |
| KX856083.1|Pseudomonas koreensis strain K5 | KX856079.1|Pseudomonas sp. Strain E10 | 0.093 | 0.0083 |
| KX856083.1|Pseudomonas koreensis strain K5 | KX856078.1|Pseudomonas sp. Strain E9 | 0.093 | 0.0083 |
| KX856079.1|Pseudomonas sp. Strain E10 | KX856078.1|Pseudomonas sp. Strain E9 | 0 | 0 |
| KX856083.1|Pseudomonas koreensis strain K5 | KX856075.1|Pseudomonas koreensis strain E5 | 0 | 0 |
| KX856079.1|Pseudomonas sp. strain E10 | KX856075.1|Pseudomonas koreensis strain E5 | 0.093 | 0.0083 |
| KX856078.1|Pseudomonas sp. strain E9 | KX856075.1|Pseudomonas koreensis strain E5 | 0.093 | 0.0083 |
| KX856083.1|Pseudomonas koreensis strain K5 | KX856072.1|Pseudomonas koreensis strain E2 | 0.2305 | 0.0147 |
| KX856079.1|Pseudomonas sp. strain E10 | KX856072.1|Pseudomonas koreensis strain E2 | 0.2553 | 0.0152 |
| KX856078.1|Pseudomonas sp. Strain E9 | KX856072.1|Pseudomonas koreensis strain E2 | 0.2553 | 0.0152 |
| KX856075.1|Pseudomonas koreensisstrainE5 | KX856072.1|Pseudomonas koreensis strain E2 | 0.2305 | 0.0147 |
| KX856082.1|Providencia sp. Strain K3 | KX856076.1|Providencia rettgeri strain E6 | 0.1301 | 0.0108 |
| KX856082.1|Providencia sp. strain K3 | KX856074.1|Providencia rettgeri strain E4 | 0.1707 | 0.0129 |
| KX856076.1|Providencia rettgeri strain E6 | KX856074.1|Providencia rettgeri strain E4 | 0.1599 | 0.012 |
| KX856082.1|Providencia sp.StrainK3 | KX856073.1|Providencia rettgeri strain E3 | 0 | 0 |
| KX856076.1|Providencia rettgeri strain E6 | KX856073.1|Providencia rettgeri strain E3 | 0.1301 | 0.0108 |
| KX856074.1|Providencia rettgeri strain E4 | KX856073.1|Providencia rettgeri strain E3 | 0.1707 | 0.0129 |
| KX856090.1|Rhizobium sp. strain S6 | KX856088.1|Rhizobium sp. Strain S4 | 0.1375 | 0.0115 |
| KX856090.1|Rhizobium sp. strain S6 | KX856086.1|Rhizobium sp. Strain S2 | 0.0609 | 0.0074 |
| KX856088.1|Rhizobium sp. strain S4 | KX856086.1|Rhizobium sp. Strain S2 | 0.128 | 0.0108 |
Table 2: Estimates of evolutionary distance among members of the same genus.
Discussion
Genetic diversity and distribution of nodule associated bacteria
This result supports other studies that found more than one species of rhizobacteria in the nodules of various leguminous plants. Stajković et al. [37] reported the isolation of 115 bacterial strains from 15 nodules, of which almost 60% were rhizobia while the rest belonged to several other genera. According to the results reported by Rajendran et al. [19] about 10% of the surface sterilized nodules tested showed the presence of endophytic nonrhizobial flora and some nodules showed more than one morphologically distinct nonrhizobial colonies. Kuklinsky-Sobral et al. [38] who reported the isolation of nodule endophytes belonged to the genera Phyllobacterium , Sphingomonas, Rhodopseudomonas, Pseudomonas, Microbacterium, Mycobacterium, and Bacillus from soya bean nodules. Costa et al. [39] isolated the genera Agromyces, Bacillus, Brevibacillus, Delfitia, Dietzia, Enterobacter, Methylobacterium, Microbacterium, Micrococcus,Paenibacillus, Pseudomonas, Rhizobium, Rhodococcus, Sphingobacterium , and Stenotrophomonas from Phaseolus vulgaris . Probably all the organisms whose presence has a beneficial relation might get associated with the plant nodules.
Pseudomonas sp. was distributed in the whole of Western Kenya region because it was isolated from all the nodule samples of common beans collected from the slopes of Mt. Elgon, shores of Lake Victoria at Kisumu and Kakamega, Its population was high in nodules from the common beans grown in Kakamega. This is an indication that it's the best-adapted nodule associated bacteria in this region. Owing to its importance as plant growth promoting bacteria [40], more sensitive characterization techniques are required to determine the type of species found in Western Kenya. Rhizobia sp. was isolated from the common beans grown on the slopes of Mt. Elgon and shores of Lake Victoria at Kisumu, but it was not isolated from those from Kakamega. Although there were very few plants with nodules from this region, nodulation has always been believed to be the reserve of rhizobia [41,42] this, therefore, calls for further studies on all other nodule associated bacteria with the aim of finding out if other bacteria apart from rhizobia are also capable of inducing nodulation. The population of rhizobia was high in Kisumu soils; in fact, it was the most abundant species of rhizobacteria in Kisumu soils. Enterobacter sp. was isolated in Kakamega and Mt. Elgon soils but not Kisumu soils and its population was highest and most abundant in Kakamega soils. Providencia sp. was isolated in Kakamega and Mt. Elgon soils and abundantly in Mt. Elgon soils. Klebsiella sp. was isolated in Mt. Elgon and Kakamega soil with similar abundance. Delfitia sp. was isolated from Kisumu and Kakamega soils and abundantly in Kisumu soils. Sphingobacterium sp. and Acinetobacter sp. was isolated only from Kisumu soils with similar abundance.
Phylogenetic analysis on the basis of 16SrRNA gene sequences provided better understanding in the evaluation of genetic diversity of NAB isolated in this study. The neighbor-joining tree constructed put the isolates into two main clusters, the second cluster was further subdivided into six other sub-clusters. Even most isolates in the same sub-cluster differed in their genetic distances showing that most of the nodule associated bacteria in the soils of Western Kenya are genetically different.
16SrRNA gene of the isolates was highly conserved but with variable regions which make it a good marker in studying evolutionary diversity. This is in tandem with other studies which have shown that the 16SrRNA gene is efficient in defining the genera because it is conserved but have variable regions, just enough to determine genetic diversity in organisms [43]. However, it has limitations in identifying species, due to the possible occurrence of genetic recombination and horizontal gene transfer resulting in sequence mosaicism [44,45], and perhaps this might be the reason why members of different genera clustered together on the phylogenetic tree. Another limitation of identifying bacteria based on the analysis of 16SrRNA genes is that species that are closely related may not always be differentiated because of the sequence conservation of 16SrRNA gene [46]. To overcome these difficulties, the use of other genes including proteincoding genes with greater sequence divergence than 16SrRNA genes, are recommended as alternative genetic markers for identification of the nodule associated bacteria [46].
Conclusion
Common bean nodule associated bacteria in Western Kenya soils are genetically diverse as shown by 16SrRNA phylogenetic analysis. This might be due to different climatic conditions experienced in the region. More studies are therefore recommended to determine their growth promotion ability in order to develop inoculants that are adapted to this region.
Declarations
The authors declare that they have no competing interests.
Funding
The project was funded by Interuniversity Council for East Africa/The Lake Victoria Research initiative and Sweden International Agency.
Acknowledgements
We are grateful to Mr. Peter Nyongesa, Mr. Willy Akanyanya, Mr. Nicholas Kitungulu and Ms. Anjeline Pamba for the technical assistance.
References
- Makalle AM, Obando J, Bamutaze Y (2008) Effects of land use practices on livelihoods in the transboundary sub-catchments of the Lake Victoria Basin. African J Environ Sci Techn 2: 309-317.
- Kawaka F, Dida MM, Opala PA, Ombori O, Maingi J, et al. (2014) Symbiotic efficiency of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in soils of Western Kenya. Intern Schol Res Not 2014.
- Savci S (2012) An agricultural pollutant: inorganic fertilizer. International Journal of Environmental Science and Development 3: 73.
- Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Resource Prot 2: 432-448.
- Osoro NO, Kawaka F, Naluyange V, Ombori O, Muoma JO, et al. (2014) Effects of water hyacinth (Eichhornia crassipes [mart.] solms) compost on growth and yield of common beans (Phaseolus vulgaris) in Lake Victoria Basin. Eur Int J Sc Tech 3: 173-186.
- Kloepper JW, Schroth MN, Miller TD (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70: 1078-1082.
- Chen C, Bauske EM, Musson G, Rodriguezkabana R, Kloepper JW (1995) Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biol Control 5: 83-91.
- Figueiredo MVB, Martinez CR, Burity HA, Chanway CP (2008) Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J Microbiol Biotechnol 24: 1187-1193.
- Acuña JJ, Jorquera MA, Martínez OA, Menezes-Blackburn D, Fernández MT, et al. (2011) Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J Soil Sci Plant Nutr 11: 1-12.
- Martyniuk S, Oron J, Martyniuk M (2005) Diversity and numbers of root-nodule bacteria [Rhizobia] in Polish soils. Acta Soc Bot Pol 74: 83-86.
- Maingi JM, Gitonga NM, Shisanya CA, Hornetz B, Muluvi GM (2006) Population levels of indigenous Bradyrhizobia nodulating promiscuous soybean in two Kenyan soils of the semi-arid and semi-humid agroecological zones. J Agr Rural Dev Trop Journal 107: 149-159
- Mwendaa GM, Karanjac NK, Bogaa H, Kahindib JHP, Muigaia A, et al. (2011) Abundance and Diversity of Legume Nodulating Rhizobia in Soils of Embu District. Tropical and Subtropical Agroecosystems 13: 1-10.
- Rodrı́guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17: 319-339.
- Khan AA, Jilani G, Akhtar MS, Naqvi SMS, Rasheed M (2009) Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. Res J Agric Biol Sci 1: 48-58.
- Fraire-Velázquez S, Rodríguez-Guerra R, Sánchez-Calderón L (2011) Abiotic and biotic stress response crosstalk in plants. Abiotic Stress Response in Plants-Physiological, Bioinorganic and Genetic Perspectives 3-26.
- Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35: 1044-1051.
- Hung PQ, Annapurna K (2004) Isolation and characterization of endophytic bacteria in soybean (Glycine sp.). Omonrice 12: 92-101.
- Wekesa CS, Okun D, Juma K, Shitabule D, Okoth P, et al. (2016) Abundance and Symbiotic Potential of Common Bean (Phaseolus vulgaris) Nodule Associated Bacteria in Western Kenya Soil. MAYFEB Journal of Agricultural Science 1: 1-9.
- Rajendran G, Patel MH, Joshi SJ (2012) Isolation and characterization of nodule-associated Exiguobacterium sp. from the root nodules of Fenugreek (Trigonella foenum-graecum) and their possible role in plant growth promotion. Int J Microbiol 2012: 1-8.
- Hamaoui B, Abbadi J, Burdman S, Rashid A, Sarig S, et al. (2001) Effects of inoculation with Azospirillum brasilense on chickpeas (Cicer arietinum) and faba beans (Vicia faba) under different growth conditions. Agronomie 21: 553-560.
- Schwartz AR, Ortiz I, Maymon M, Herbold CW, Fujishige NA, et al. (2013) Bacillus simplex—a little known PGPB with anti-fungal activity—alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv viciae. Agronomy 3: 595-620.
- Anyango B, Wilson KJ, Beynon JL, Giller KE (1995) The diversity of rhizobia nodulating Phaseolus vulgaris L. in two Kenyan soils with contrasting pHs. Appl Environ Microbiol 61: 4016-4021.
- Rastogi G, Sani RK (2011) Molecular techniques to assess microbial community structure, function, and dynamics in the environment. Microbes and Microbial Technology 29-57.
- Olsen GJ, Lane DJ, Giovannoni SJ, Pace NR, Stahl DA (1986) Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol 40: 337-365.
- Das AJ, Kumar M, Kumar R (2013) Plant growth promoting rhizobacteria (PGPR): an alternative of inorganic fertilizer for sustainable, environment friendly agriculture. Res J Agriculture & Forestry Sci 1: 21-23.
- Issar S, Sharma S, Choudhary DK, Gautam HK, Gaur RK (2012) Molecular characterization of Pseudomonas spp. isolated from root nodules of various leguminous plants of Shekhawati Region, Rajasthan, India. AJPS 3: 60.
- Mahbouba B, Nadir B, Nadia Y, Abdelhamid D (2013) Phenotypic and molecular characterization of plant growth promoting Rhizobacteria isolated from the rhizosphere of wheat (Triticum durum Desf.) in Algeria. Afr J Microbiol Res 7: 2893-2904.
- Vinay O, Bhupendra P, Kiran S (2013) 16S rDNA-RFLP analysis of phylogenetic tree of Rhizobium bacteria. IJAR 3: 474-476.
- Liu L, Li Y, Li S, Hu N, He Y, et al. (2012) Comparison of next-generation sequencing systems. BioMed Res. Int 2012: 1-11.
- Rincón A, Arenal F, González I, Manrique E, Lucas MM, et al. (2008) Diversity of rhizobial bacteria isolated from nodules of the gypsophyte Ononis tridentata L. growing in Spanish soils. Microb Ecol 56: 223-233.
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95-98.
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res 22: 4673-4680.
- Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406-425.
- Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783-791.
- Jukes TH, Cantor CR (1969) Evolution of protein molecules. In Munro HN, editor, Mammalian Protein Metabolism 21-132.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725-2729.
- Stajković O, Delić D, Jošić D, Kuzmanović Đ, Rasulić N, et al. (2011) Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom Biotechnol Lett 16: 5919-5926.
- Kuklinsky-Sobral J, Araujo WL, Mendes R, Pizzirani-Kleiner AA, Azevedo JL (2005) Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil 273: 91-99.
- Costa LEDO, Queiroz MVD, Borges AC, Moraes CAD, et al. (2012) Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz J Microbiol 43: 1562-1575.
- Yadegari M (2014) Inoculation of bean (Phaseolus vulgaris) seeds with Rhizobium phaseoli and plant growth promoting Rhizobacteria. Adv Environ Biol 419-425.
- Fonseca MB, Peix A, de Faria SM, Mateos PF, Rivera LP, et al. (2012) Nodulation in Dimorphandra wilsonii Rizz. (Caesalpinioideae), a threatened species native to the Brazilian Cerrado. PloS one 7: e49520.
- Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156: 567-576.
- Silva FV, Simões-Araújo JL, Silva Júnior JP, Xavier GR, Rumjanek NG (2012) Genetic diversity of Rhizobia isolates from Amazon soils using cowpea (Vigna unguiculata) as the trap plant. Braz J Microbiol 43: 682-691.
- Munns DN, Keyser HH (1981) Response of Rhizobium strains to acid and aluminium stress. Soil Biol Biochem 13: 115-118.
- Ntushelo K (2013) Identifying bacteria and studying bacterial diversity using the 16S ribosomal RNA gene-based sequencing techniques: A review. Afr J Microbiol Res 7: 5533-5540.
- Martens M, Delaere M, Coopman R, De Vos P, Gillis M, et al. (2007) Multilocus sequence analysis of Ensifer and related taxa. Int J Syst Evol Microbiol 57: 489-503.
- Peters JB, Laboski CA, Bundy LG (2007) Sampling soils for testing. Division of Cooperative Extension of the University of Wisconsin—Extension 2100.
Copyright: © 2017 Wekesa CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.