International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Original Research Article - (2022)
Background: Advanced Glycation End products (AGEs) has been identified as a significant source of neurotoxicity in Alzheimer's Disease (AD). There is growing evidence confirming that many medicinal plants and natural compounds can potentially lessen the progression of neurodegenerative disorders or avert the onset of neurodegeneration, thus attracting much attention. Gigantol, a biphenyl phenolic compound isolated from orchids of the genus Dendrobium, has been reported to halt high glucose-induced renal dysfunction. Based on these data, gigantol has been proposed as a preventive treatment against cells from hyperglycemia or glycation-associated damages; however, there is no comprehensible evidence. AGEs induced damage in SH-SY5Y neuroblastoma cells was used to clarify the beneficial effects of gigantol on AD.
Methods and findings: Before stimulation with AGEs, SH-SY5Y cells were pretreated with gigantol. Gigantol (25 μmol/L) increased the cell viability reduced by AGEs (50 μg/mL); it also attenuated AGEs-induced reactive oxygen species generation and ameliorated downregulation of superoxide dismutase, glutathione peroxidase, and catalase. We found that AGEs increased Amyloid-Beta (Aβ) secretion in parallel with Amyloid Precursor Protein (APP) upregulation was reversed by gigantol. Gigantol did not affect β-site APP-cleaving enzyme 1 expression but induced insulin-degrading enzyme and neprilysin expression, which promoted degradation of Aβ. Gigantol also decreased the AGEs-induced protein levels of Endoplasmic Reticulum (ER) stress-associated molecules, including 78-kDa glucoseregulated protein and C/EBP homologous protein, and lowered phosphorylation of protein kinase R-like endoplasmic reticulum kinase and activating transcription factor 4. The neuro apoptosis effects caused by AGEs, such as upregulation of Bax, active caspase 12, cleaved caspase 3, and downregulation of Bcl-2, were alleviated by gigantol.
Conclusion: Gigantol could counteract AGEs related adverse effects by modulating Aβ degradation and inhibiting ER stress-associated apoptosis. Gigantol could be a potential therapeutic compound to halt or cure AD.
Advanced glycation end products; Alzheimer's disease; Gigantol; Endoplasmic reticulum; SH-SY5Y cells
Accumulated evidence suggests that diabetic subjects are at an increased risk for Alzheimer's Disease (AD), a neurodegenerative disease with progressive memory cognitive dysfunction [1]. The pathology associated with diabetes-mediated neurodegeneration involves chronic hyperglycemia, which accelerates the reaction between glucose and proteins and leads to advanced glycation end products (AGEs) [2]. AGEs can interact with the cell surface receptors (RAGE), leading to an increase in the expression of Amyloid Precursor Protein (APP) and Amyloid-Beta (Aβ) via reactive oxygen species [3]. Deficiencies in Aβ clearance or enzyme-mediated Aβ degradation are thought to lead to accumulation and aggregation of Aβ, which interfere with several cellular processes and result in the Endoplasmic Reticulum (ER) stress to trigger a complex multistep cascade leading to dementia [4-6]. ER stress uncouples the interaction between the 78-kDa Glucose-Regulated Protein (GRP78) and ER stress sensors, which triggers activation of the Unfolded Protein Response (UPR) branches leading to restore ER homeostasis [7]. However, UPR fails to fix the ER's normal function during prolonged or overwhelming ER stress and can trigger pro-apoptotic signals by activating the transcription factor C/EBP homologous protein (CHOP) [8]. CHOP acts to repress the B-cell lymphoma 2 (Bcl-2) gene expression; thus, downregulating anti-apoptotic Bcl-2 protein, but has been shown to promote activation and translocation of pro-apoptotic proteins such as Bcl-2-associated X protein (Bax) to mitochondria [8]. In addition to mediating by a transcription factor-dependent pathway, the UPR can elicit apoptosis by another mechanism, a caspase-dependent path [9]. Caspase-12 plays a vital role in carrying out the caspase-dependent UPR. Upon activation of the UPR, caspase-12 translocates from the ER to the cytosol and caspase-9, which cleaves and activates effector caspase-3 [9]. Therefore, it is considered that block of the AGE/RAGE signaling-mediated ER stress would be beneficial therapeutic modalities in the prevention, regression, and slowing of neurodegenerative disease progression, including AD [1].
There is a growing need for medications to treat AD progression neurodegenerative events due to its complex nature. However, the multitude of adverse responses reported from the current synthetic drugs is the leading cause of failure in drug development to treat or halt AD progression and mandate the search for safer and more efficient alternatives [10]. Many medicinal plants and natural compounds have potential adjunctive therapeutic effects on AD and memory deficits due to their ability to lessen the progression of neurodegenerative disorders or avert the onset of neurodegeneration, thus attracting much attention [11,12].
Gigantol (3',4-dihydroxy-3,5'-dimethoxybibenzyl) is a biphenyl phenolic compound isolated from orchids of the genus Dendrobium [13]. This biologically active compound has been reported to possess several bioactions, including anti-osmotic, antioxidant, antispasmodic, antinociceptive, and antiinflammatory [14-16]. Gigantol displays an antitumoral activity against breast cancer and lung cancer [17,18]. Gigantol and its derivatives have been described as potent compounds to prevent diabetic cataracts [19]. Additionally, gigantol halts high glucoseinduced renal dysfunction has been documented [20]. Based on these data, gigantol has been proposed as a preventive treatment against cells from hyperglycemia or glycation-associated damages; however, there is no comprehensible evidence relating to its protective role in the neurodegenerative diseases augmented under conditions of glycation.
Human neuroblastoma SH-SY5Y cells can be differentiated from a neuroblast-like state into mature human neurons and have also been utilized in models for neuropathy wherein the effects of high glucose and AGEs [21]. Thus, the SH-SY5Y cells line model system is widely used for investigating and assessing the neuroprotective effects of natural compounds against the models of neurodegenerative disease [22]. In the present study, AGEs-induced injury in SH-SY5Y neuronal cells was used as an in vitro neurodegenerative disease to clarify the beneficial effects of gigantol on the neurodegenerative diseases augmented under glycation conditions.
Cell culture and treatments
The SH-SY5Y cells (no. CRL-266) purchased from American Type Culture Collection (Manassas, VA, USA) were cultured in growth media composed of Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 1% antibiotics (streptomycin and penicillin) for 24 h to allow for adherence. Following adherence, the growth media was replaced with a differentiation media DMEM supplemented with 1% FBS and 10 μmol/L retinoic acid to induce the neuronal differentiation of SH-SY5Y cells in five days of culture. Treatments for all experiments took place following the five-day differentiation period. For AGEs-induced functional studies, cells were maintained in a fresh medium containing 1% FBS for 2 h before the experiments. Later, cells were pretreated with gigantol (Chengdu Chroma-Biotechnology Co., Ltd., Chengdu Shi, Sichuan, China, purity ≥ 98%) at different concentrations, or N-Acetyl-L-Cysteine (NAC, Sigma-Aldrich; purity, ≥ 99%) at 1 mmol/L, for 1 h followed by exposure to various concentrations of AGEs in Bovine Serum Albumin (AGEs-BSA) (Sigma- Aldrich) for different time points without medium change. The dosage regimen of gigantol was selected based on a previous report demonstrating that this compound at these concentrations significantly protected the mouse glomerulus mesangial cell from high glucose-evoked injury without cytotoxicity [20].
Cell viability measurements
Cell viability was determined by a 3-(4,5-dimethyl thiazol-2- yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) colorimetric assay [23]. The absorbance of the samples was measured at 570 nm on a microplate reader (SpectraMax M5, Molecular Devices, USA), and background optical densities were subtracted from that of each well. The net absorbance from the plates of cells cultured with the control medium (not treated) was considered 100% cell viability.
Measurement of intracellular ROS levels and antioxidant enzyme activities
The ROS production was measured with Dichloro-Dihydro- Fluorescein Diacetate (DCFH-DA; Sigma-Aldrich), following the manufacturer's instructions. The fluorescence intensity was measured using a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) at excitation and emission wavelengths of 488 and 530 nm, respectively [24]. Estimations of antioxidant enzymes, such as Super-Oxide Dismutase (SOD), Glutathione Peroxidase (GPx), and Catalase (CAT), were performed using a SOD Activity Colorimetric Assay Kit (Cat. #K335), GPx Colorimetric Assay Kit (Cat. #K762), and CAT Activity Colorimetric Assay Kit (Cat. #K773), respectively, following the procedures provided by Bio Vision, Inc. (San Francisco, CA, USA). Enzyme activities were calculated using absorption (450 nm for SOD, 340 nm for GPx, and 570 nm for CAT) and expressed as units per milligram protein. The protein concentration was measured by using a Bio-Rad protein assay.
Measurement of Aβ1-40 and Aβ1-42
Cell medium was collected and centrifuged at 12,000 rpm for 15 min at 4°C. The supernatants were collected to measure the amount of Aβ1-40 (Cat. #KHB3481) and Aβ1-42 (Cat. #KHB3441) by ELISA assay kits following the manufacturer’s instructions (Invitrogen, California, USA). Optical density was measured at 450 nm using a microplate reader (Spectramax M5). The results were calculated from a standard curve.
Measurement of caspases-12, -9, and -3 activities
The caspases-12-like activity was measured using a fluorometric kit (Cat. # ab65664, Abcam plc., Cambridge, UK). The activities of caspases-9 (Cat. #ab65608, Abcam plc.) and -3 (Cat. #ab39401, Abcam plc.) were measured by the colorimetric assay kits. A fluorometer quantified the caspases-12-like activity by fluorescent detection of free AFC after cleaving from the peptide substrate ATAD-AFC at Ex/Em=400/505 nm. For caspases-9 and -3 activities measurement, aliquots of cellular extracts containing 200 μg of protein were reacted with 200 μmol/L Ac-LEHD-pNA (chromogenic substrate for caspase-9) or Ac-DEVD-pNA (chromogenic substrate for caspase-3), in reaction buffer for 2 h at 37°C. A microplate reader determined the caspase-9/-3-like activity by measuring substrate cleavage at 405 nm. Results were expressed relative to the untreated control.
Quantification of DNA fragmentation
The cell death detection ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany) was used to quantitatively detect the cytoplasmic histone-associated DNA fragments (mono and oligo nucleosomes) after induced cell death [25]. Quantification of histone-associated DNA fragments was determined at a wavelength of 405 nm by using a microplate reader (Spectramax M5). The optical density at 405 nm reading was normalized to each sample's total amount of protein. Data are normalized to the untreated control.
Western blot analysis
After treatments, the cells were collected and homogenized in lysis buffer containing a mixture of 30 mmol/L Tris-HCl, pH 7.4, 250 mmol/L Na3VO4, 5 mmol/L EDTA, 250 mmol/L sucrose, 1% Triton X-100 with protease inhibitor and phosphatase inhibitor cocktail. The total protein sample was loaded into 10% Sodium Dodecyl Sulfate-Poly Acrylamide Gel Electrophoresis (SDS PAGE) loading buffer before being transferred to Poly Vinylidene Di-Fluoride (PVDF) membranes, which were sealed with 5% skim milk at 37ºC for 120 min. After blocking with 5% BSA for 1 h at room temperature, the membranes were then incubated overnight at 4ºC with the following primary antibodies: APP (Cell Signaling Technology, Beverly, CA, USA, #2452), Aβ (Santa Cruz Biotechnology, Inc., #sc-28365), β-site APP-cleaving enzyme 1 (BACE1; Santa Cruz Biotechnology, Inc., sc-33711), insulin-degrading enzyme (IDE; Santa Cruz Biotechnology, Inc., #sc-393887), Neprilysin (NEP; Santa Cruz Biotechnology, Inc., #sc-9149), GRP78 (Santa Cruz Biotechnology, Inc., #sc-13539), Protein kinase-like Endoplasmic Reticulum Kinase (PERK; Cell Signaling Technology, #5683), p- PERK (Thr980) (Cell Signaling Technology, #3179), eukaryotic initiation factor 2α (p-eIF2α; Cell Signaling Technology, #5324), p-eIF2a (Ser51) (Cell Signaling Technology, #9721), activating transcription factor 4 (ATF4; Cell Signaling Technology, #11815), CHOP (Cell Signaling Technology, #2895). The β-actin antibody (Santa Cruz Biotechnology, Inc., #sc-8432) was used as an internal control in immunoblotting. All antibodies were utilized at 1:1000 dilution. After the membranes were washed three additional times, the membranes were incubated with a horseradish peroxidase secondary antibody (1:4000) for one hour on a shaker at room temperature. Immunoreactive bands were then visualized using an enhanced chemiluminescence detection kit (Amersham Biosciences, Buckinghamshire, UK). Finally, the relative amount of immunoreactive protein in each band was measured by scanning densitometric analysis using the ATTO Densitograph Software (ATTO Corp., Tokyo, Japan) and quantified as the ratio to β-actin. The mean value for samples from the control medium (not treated) on each immunoblot, expressed in densitometry units, was adjusted to 1.0. All experimental sample values were then expressed relative to this adjusted mean value. Cells were sampled from five independent experiments.
Statistical analysis
Data are expressed as the mean ± Standard Deviation (SD) of five independent experiments (n=5) performed in triplicate. Statistically-significant differences were evaluated by one-way analysis of variance and Dunnett range post-hoc comparisons using Systat SigmaPlot version 12.5 (Systat Software Inc., San Jose, CA, USA. Differences were considered statistically significant at p<0.05.
Gigantol alleviates decreased cell viability in AGEstreated SH-SY5Y cells
When SH-SY5Y cells were incubated with AGEs at concentrations from 5 to 100 μg/mL for 24 hours, the cell viability reduced from 80.3% to 51.9%, respectively; Figure 1A. Moreover, AGEs (50 μmol/L) reduced the cell viability in SHSY5Y cells in a time-dependent tendency; Figure 1B. In this study, SH-SY5Y cells were exposed to 50 μmol/L AGEs for 24 hours to induce neuronal insults in the following experiments.
Gigantol pretreatment for 1 h before AGEs (50 μmol/L) exposure improved cell viability associated with an increasing concentration of gigantol (10-25 μmol/L); the protective potency was most potent at the 25 μmol/L concentration; Figure 1C. Pretreatment gigantol (25 μmol/L) sustains the survival rate of the AGEs-treated SH-SY5Y cells to 91.2%, and similar results were obtained in AGEs (50 μmol/L)-cultured cells treated with 1 mmol/L of NAC; Figure 1C. As shown in Figure 1D, neither gigantol nor NAC alone affects cell death.
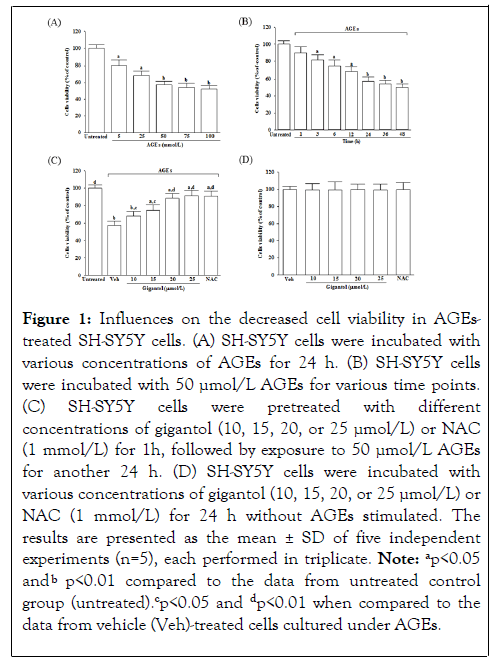
Figure 1: Influences on the decreased cell viability in AGEstreated SH-SY5Y cells. (A) SH-SY5Y cells were incubated with various concentrations of AGEs for 24 h. (B) SH-SY5Y cells were incubated with 50 µmol/L AGEs for various time points. (C) SH-SY5Y cells were pretreated with different concentrations of gigantol (10, 15, 20, or 25 µmol/L) or NAC (1 mmol/L) for 1h, followed by exposure to 50 µmol/L AGEs for another 24 h. (D ) SH-SY5Y cells were incubated with various concentrations of gigantol (10, 15, 20, or 25 µmol/L) or NAC (1 mmol/L) for 24 h without AGEs stimulated. The results are presented as the mean ± SD of five independent experiments (n=5), each performed in triplicate. Note: ap<0.05 and bp<0.01 compared to the data from untreated control group (untreated).cp<0.05 and dp<0.01 when compared to the data from vehicle (Veh)-treated cells cultured under AGEs.
Influences on oxidative stress and antioxidant status in AGEs-treated SH-SY5Y cells
In AGEs cultured SH-SY5Y cells, the intracellular ROS production was 2.3-fold higher than in untreated controls, reduced by gigantol pretreatment concentration-dependent; Figure 2A. Gigantol (25 μmol/L) pretreatment reduced the AGEs-induced ROS generation in SH-SY5Y cells by 34.6%; the results were similar to the effects produced by NAC 1 mmol/L; Figure 2A.
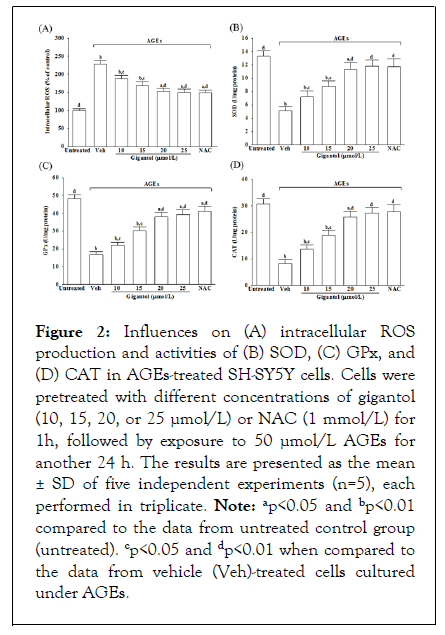
Figure 2: Influences on (A) intracellular ROS production and activities of (B) SOD, (C) GPx, and (D) CAT in AGEs-treated SH-SY5Y cells. Cells were pretreated with different concentrations of gigantol (10, 15, 20, or 25 µmol/L) or NAC (1 mmol/L) for 1h, followed by exposure to 50 µmol/L AGEs for another 24 h. The results are presented as the mean ± SD of five independent experiments (n=5), each performed in triplicate. Note: ap<0.05 and bp<0.01 compared to the data from untreated control group (untreated). cp<0.05 and dp<0.01 when compared to the data from vehicle (Veh)-treated cells cultured under AGEs.
It markedly decreased in the activities of SOD and GPx when SH-SY5Y cells were incubated with 50 μmol/L AGEs for 24 h; gigantol pretreatment abrogated these situations by a concentration-dependent manner; Figures 2B and 2C. NAC (1 mmol/L) pretreatment with AGEs-cultured SH-SY5Y cells reversed the reductions in SOD and GPx activities with results similar to those produced by gigantol 25 μmol/L; Figures 2B and 2C. The CAT activity in SH-SY5Y cells cultured in AGEs medium was lower to 26.8% than the untreated control group; Figure 2D. Pretreatment of SH-SY5Y cells with gigantol (25 μmol/L) or NAC (1 mmol/L) resulted in an increase of CAT activity to 3.3 and 3.4-fold, respectively, relative to those observed in the vehicle-treated counterparts.
Effects on APP processing and Aβ secretion in AGEs-treated SH-SY5Y cells
Exposure of SH-SY5Y cells to AGEs significantly increased APP protein levels, which was decreased by 69.4% when cells received pretreatment with 25 mmol/L gigantol; Figure 3A. The intracellular levels of BACE1 in SH-SY5Y cells cultured in AGEs medium were higher by 4.4-fold than the vehicle-treated group; Figure 3A. However, there were no changes in protein levels of BACE1in cell pretreatment with gigantol compared to AGEs treatment alone; Figure 3A. The protein levels of IDE and NEP in AGEs cultured SH-SY5Y cells were lower to 43.3% and 45.7% of those in untreated controls, respectively, while gigantol pretreatment resulted in upregulation of both protein levels of IDE and NEP; Figure 3A.
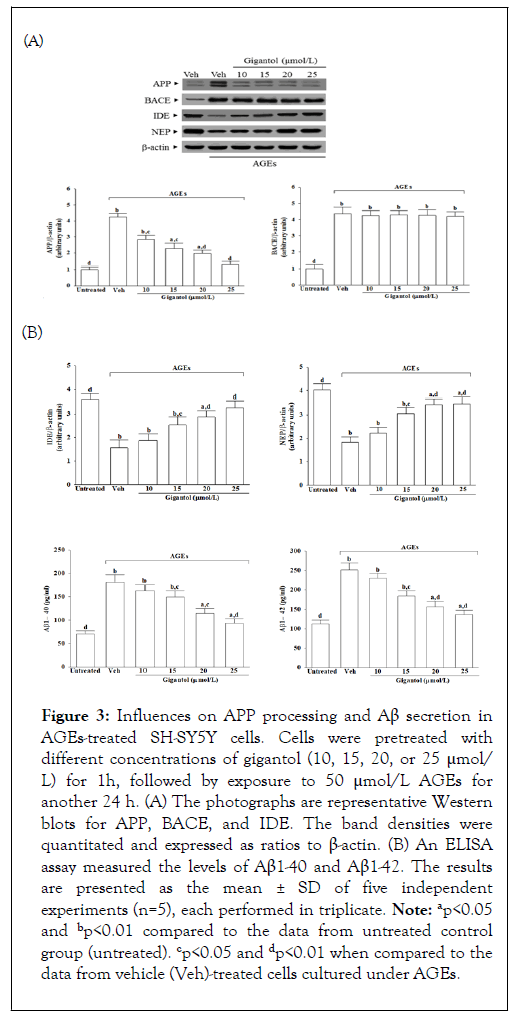
Figure 3: Influences on APP processing and Aβ secretion in AGEs-treated SH-SY5Y cells. Cells were pretreated with different concentrations of gigantol (10, 15, 20, or 25 μmol/ L) for 1h, followed by exposure to 50 μmol/L AGEs for another 24 h. (A) The photographs are representative Western blots for APP, BACE, and IDE. The band densities were quantitated and expressed as ratios to β-actin. (B) An ELISA assay measured the levels of Aβ1-40 and Aβ1-42. The results are presented as the mean ± SD of five independent experiments (n=5), each performed in triplicate. Note: ap<0.05 and bp<0.01 compared to the data from untreated control group (untreated). cp<0.05 and dp<0.01 when compared to the data from vehicle (Veh)-treated cells cultured under AGEs.
It showed that AGEs caused 2.6-fold and 2.2-fold increases in levels of Aβ1-40 and Aβ1-42 in SH-SY5Y cells, respectively, whereas gigantol pretreatment attenuated these enhancements in a concentration-dependent manner; Figure 3B. The level of Aβ1-40 and Aβ1-42 significantly decreased to 51.6% and 54.1%, respectively, in AGEs-cultured SH-SY5Y cells with gigantol (25 μmol/L) pretreatment relative to the levels in their vehicletreated counterparts.
Influences on the ER stress-relevant molecules in AGEs-treated SH-SY5Y cells
The protein level of GRP78 in SH-SY5Y cells under AGEs stimulated elevated to 4.4-fold of the regular group; Figure 4. Gigantol (25 μmol/L) pretreatment lowered the GRP78 protein level in AGEs-cultured SH-SY5Y cells by 52.6% of the vehicletreated counterpart group; Figure 4.
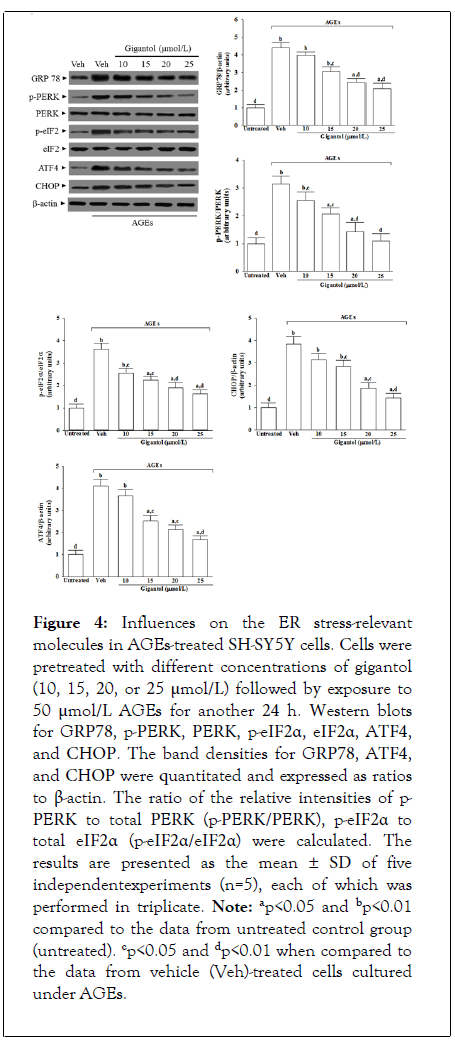
Figure 4: Influences on the ER stress-relevant molecules in AGEs-treated SH-SY5Y cells. Cells were pretreated with different concentrations of gigantol (10, 15, 20, or 25 μmol/L) followed by exposure to 50 μmol/L AGEs for another 24 h. Western blots for GRP78, p-PERK, PERK, p-eIF2α, eIF2α, ATF4, and CHOP. The band densities for GRP78, ATF4, and CHOP were quantitated and expressed as ratios to β-actin. The ratio of the relative intensities of p- PERK to total PERK (p-PERK/PERK), p-eIF2α to total eIF2α (p-eIF2α/eIF2α) were calculated. The results are presented as the mean ± SD of five independentexperiments (n=5), each of which was performed in triplicate. Note: ap<0.05 and bp<0.01 compared to the data from untreated control group (untreated). cp<0.05 and dp<0.01 when compared to the data from vehicle (Veh)-treated cells cultured under AGEs.
The immunoblot results showed that the ratio of phosphoprotein to total protein in PERK (p-PERK/PERK) and eIF2α (p-eIF2α/eIF2α) were 3.1 and 3.6-fold more significant in the AGEs cultured SH-SY5Y cells than those in the vehicletreated control group, respectively; Figure 4. The pretreatment of AGEs cultured SH-SY5Y cells with gigantol (25 μmol/L) downregulated p-PERK/PERK and p-eIF2α/ eIF2α to 1.1 1.6-fold to those in their vehicle-treated counterpart, respectively; Figure 4. Whether in the presence of gigantol or not, the expression of total PERK and eIF2α protein did not change in SH-SY5Y cells stimulated with AGEs; Figure 4.
Immunoblot analysis showed that the protein levels of ATF4 and CHOP were 4.1 and 3.8-fold higher in the AGEs cultured SH-SY5Y cells than in the vehicle-treated control group, respectively; Figure 4. AGEs-induced upregulation on ATF4 and CHOP protein expression in SH-SY5Y cells remarkably reversed in gigantol (25 μmol/L) pretreatment (59.2% decreases in ATF4, 62.2% decreases in CHOP, relative to those in their vehicle-treated counterparts.
Effects on the ER stress-associated apoptosis in AGEs-treated SH-SY5Y cells
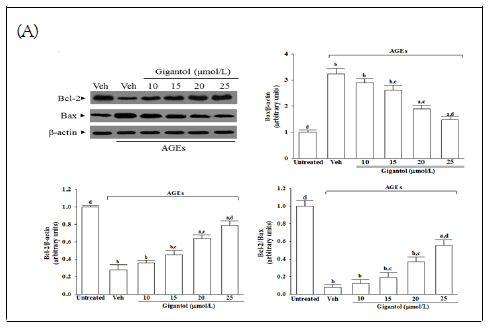
The protein expression of Bcl-2 lowered in the AGEscultured SH-SY5Y cells compared with the vehicle-treated group; gigantol abrogated the AGEs-induced downregulated Bcl-2 protein expression in a concentration-dependent manner; (Figure 5A). Gigantol concentration-dependently reversed the increased Bax detected in SH-SY5Y cells cultured under AGEs; Figure 5A. The expression ratio of Bcl-2 to Bax has significantly decreased in AGEs cultured SH-SY5Y cells; gigantol pretreatment concentration-dependently enhanced the ratio of Bcl-2 to Bax; Figure 5A.
AGEs caused a 3.3-fold increase in caspase-12-like activity in SHSY5Y cells; Figure 5B. Pretreatment SH-SY5Y cells with gigantol (25 μmol/L), the higher caspase-12-like activity caused by AGEs were lower to 48.2% of those in their vehicle-treated counterparts; Figure 5B. The caspase-like activities of caspase-9 and caspase-3 were 4.0 and 3.5 fold higher in the AGEs-cultured SH-SY5Y cells than in the vehicle-treated group; Figure 5B. In the presence of gigantol (25 μmol/L), the increased caspase-like activities of caspase-9 and caspase-3 in SH-SY5Y cells exposed to AGEs were downregulated 55.7% and 41.4%, respectively of those observed on the vehicle-treated counterparts; Figure 5B. DNA fragmentation in the AGEs-cultured SH-SY5Y cells was 3.3 fold higher relative to the vehicle-treated group, which was decreased by 62.3% in AGEs-cultured SH-SY5Y cells pretreatment with gigantol (25 μmol/L; Figure 5C.
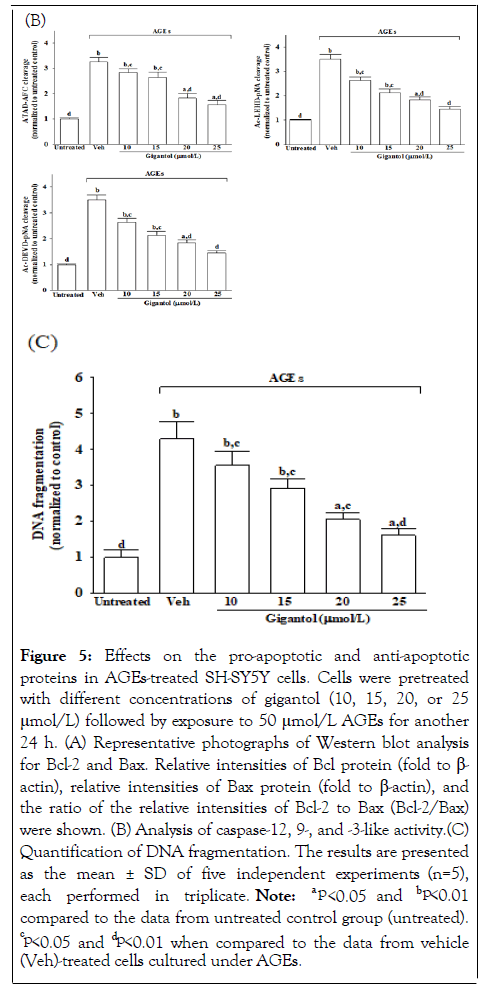
Figure 5: Effects on the pro-apoptotic and anti-apoptotic proteins in AGEs-treated SH-SY5Y cells. Cells were pretreated with different concentrations of gigantol (10, 15, 20, or 25 μmol/L) followed by exposure to 50 μmol/L AGEs for another 24 h. (A) Representative photographs of Western blot analysis for Bcl-2 and Bax. Relative intensities of Bcl protein (fold to β- actin), relative intensities of Bax protein (fold to β-actin), and> the ratio of the relative intensities of Bcl-2 to Bax (Bcl-2/Bax) were shown. (B) Analysis of caspase-12, 9-, and -3-like activity.(C) Quantification of DNA fragmentation. The results are presented as the mean ± SD of five independent experiments (n=5), each performed in triplicate. Note: a p<0.05 and bp<0.01 compared to the data from untreated control group (untreated). cp<0.05 and dp<0.01 when compared to the data from vehicle (Veh)-treated cells cultured under AGEs.
Since AGEs-induced oxidative stress significantly influences neurodegenerative disorders, a logical therapeutic approach prevents oxidative stress by increasing antioxidant defense [2]. Phytochemicals with antioxidant activity reduce oxidative stress, thus representing beneficial drug candidates for treating and preventing neurodegenerative disorders; a significant antioxidant activity of gigantol might contribute to the neuroprotective action [16, 26]. The antioxidant NAC protective against the AGEs-induced oxidative damage in SHSY5Y cells has been reported [27]; thus, NAC was used as a positive control to investigate whether the action of gigantol on the rescue cell growth was related to the amelioration of AGEs-induced ROS generation. Our data support that gigantol counteracts AGEstriggered oxidative damage in SH-SY5Y cells, contributing to a balance between oxidative stress and antioxidant defense systems. Therefore, further research is expected to examine mechanisms by which gigantol protects against AGEs-induced neuronal cellular damage.
AGEs-induced ROS increase the synthesis of Aβ through the proteolytic processing of APP by up-regulating the activity of BACE1, leading to aggregation and deposition of Aβ to cause neuronal damage has been well established [5]. Aβ is degraded by degrading enzymes, among which NEP and IDE are the most important belongs to two metalloprotease family members, M16 (IDE) and M13 (NEP), respectively [5]. Aβ occurs in different length variants with peptides of 40 amino acid residues (Aβ1-40) and 42 amino acid residues (Aβ1-42) being the most prevalent; the more extended Aβ1-42 variant has a much higher propensity to form aggregates [7]. In AGEs-cultured SH-SY5Y cells, gigantol pretreatment resulted in translational downregulation of APP accompanied by a decrease in Aβ secretion, but it did not affect BACE1 protein levels in this process. It revealed that gigantol could ameliorate Aβ overproduction not directly via inhibiting Aβ generation. Indeed, gigantol treatment resulted in an upregulation of IDE and NEP protein levels, showing that the attenuation of Aβ secretion by gigantol is mediated by modulating Aβ degradation. Therefore, these findings support the notion that gigantol may regulate Aβ degradation rather than the generation in neuronal cells.
Emerging evidence indicates that the accumulation of AGEs in human tissues may contribute to cell injury, inflammation, and apoptosis through induction of ER stress, which has mainly been implicated in the etiology and pathogenesis of multiple human diseases, including AD [28]. A significant response to ER stress is the activation of the GRP78 through dissociation from its transmembrane receptor, including PERK, IRE-1, and activating transcription factor (ATF) 6, leading to the activation of three typical signal pathways [29,30]. In contrast to the IRE1 and ATF6 signaling pathways, the PERK/eIF2α/ATF4 axis activation is sustained under prolonged ER stress conditions, thus activating the transcription of genes involved in cell apoptosis CHOP protein [31]. Several lines of evidence suggest that the pathogenesis and progression of AD are closely associated with the overactivation of the PERK-dependent UPR signaling pathway; therapeutic targeting on the PERK-mediated signaling branches may help develop a potential approach to treat AD [32]. We observed AGEs-induced upregulation of GRP78, p-PERK, p-eIF2α, and ATF4 were attenuated in the gigantol treatment group. Besides increasing CHOP expression level in SH-SY5Y cells after induction with AGEs, this effect was attenuated by gigantol pretreatment. These data suggested that the survivability of AGEs-treated SH-SY5Y cells enhanced by gigantol through suppressing the ER stress via downregulation of PERK/eIF2α/ATF4/CHOP pathway, contributing to neuron protection.
Although Bcl-2 family proteins are best characterized at the mitochondrion, they are localized to other intracellular membranes such as the ER and nuclear membranes [33]. As a transcriptional factor, CHOP has been shown to promote activation and translocation of pro-apoptotic proteins such as Bax to mitochondria and downregulates expression of Bcl-2 by repressing its promoter [8]. Gigantol prevented AGEs-induced Bcl-2 protein downregulation and lowered Bax levels in SHSY5Y cells. Therefore, gigantol-induced reductions of the AGEinduced ER stress may inhibit neuronal apoptosis, which was associated with restoring the balance between the pro-and antiapoptotic proteins of the Bcl-2 family.
During prolonged ER stress, the ER membrane resident caspase-12 can be activated subsequently triggers apoptotic pathways by cleaving and activating caspase-9 [9]. Caspase-9 activates effector caspases such as caspase-3, resulting in cleavage of cellular proteins and cell demise by apoptosis [34].
Compared with AGEs-treated SH-SY5Y cells, gigantol-pretreated cells had a minor apoptotic DNA fragmentation and decreased along with cleavage of caspase-12; protein expression and the activity of cleaved caspase-9 and -3 expressions were also downregulated by gigantol pretreatment. Our data suggest that gigantol prevents the AGEs-induced activation of SH-SY5Y cell apoptosis and that the effect may partly be regulated by cellular signaling of the caspase 12-dependent apoptotic pathway. The data presented here show the protective role of gigantol in neuroblastoma cells by its ability to counteract AGEs adverse effects via multiple mechanisms and may be a natural weapon against neurodegenerative disorders associated with glycation.
The present study was supported by a grant from the Ministry of Science and Technology (MOST 110-2320-B-127-001) of Taiwan.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lai MC, Liu WY, Liou SS, Liu IM (2022)Gigantol, A Biphenyl Phenolic Compound of Medicinal Orchids, against Amyloid β Aggravation of SH-SY5Y Neuroblastoma Cell Injury Induced by Advanced Glycation End Products. Int J Phys Med Rehabil. S16:002.
Received: 21-Mar-2022, Manuscript No. JPMR-22-16307; Editor assigned: 23-Mar-2022, Pre QC No. JPMR-22-16307 (PQ); Reviewed: 06-Apr-2022, QC No. JPMR-22-16307; Revised: 13-Apr-2022, Manuscript No. JPMR-22-16307 (R); Published: 22-Apr-2022 , DOI: 10.35248/2329-9096.22.S16.002
Copyright: © 2022 Lai MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.