Research Article - (2018) Volume 4, Issue 3
Glycerol and Phosphorus Augmentation Coupled with pH Alteration are Potential Facilitators of Petroleum Hydrocarbon Bio-diminution in Contaminated Soil
*Corresponding Author: Lekiah Pedro Peekate, Department of Microbiology, Faculty of Science, Rivers State University, P. M. B. 5080 Port Harcourt, Nigeria, Tel: +2348063353116 Email:
Keywords: Augmentation; pH; Glycerol; Phosphorus; Petroleum hydrocarbon; Bioremediation
Introduction
Bioremediation of petroleum hydrocarbon polluted soil can be achieved through the addition of easily utilizable carbon compounds that will lead to co-metabolism of the petroleum hydrocarbon molecules. Addition of glycerol, a carbon compound easily utilized by many microorganisms, has been shown to increase the biodegradation rate of high-molecular weight polycyclic aromatic hydrocarbons [1]. The degradation rates in the study suggested that the degradation of the aromatic hydrocarbons occurred through co-metabolism. High percentage removal of polycyclic aromatic hydrocarbons through the use of glycerol as a co-substrate was also obtained by the researchers in another study [2]. Addition of macronutrients and adjustment of pH can also enhance the removal of hydrocarbons in petroleum hydrocarbon polluted environment. Phosphorus as the dominant macronutrient in a nutrient mixture has been shown to enhance oxygen uptake thereby leading to enhanced bioremediation of petroleum hydrocarbon contaminated soils [3]. In another study where methane and air were initially tested for in situ bioremediation of a trichloroethylene-contaminated site, MPN values of methanotrophs increased only after addition of a gas mixture containing phosphorus [4]. This indicated that the gas mixture containing phosphorus stimulated methanotrophic biodegradation of the trichloroethylene contaminant.
Addition of phosphorus to a hydrocarbon contaminated environment will invariable follow the concept of CP ratio. The optimum CP ratio required for biodegradation of hydrocarbons in contaminated soil as implied from the review of Thapa et al. is 100:1. However, a CP ratio of 10:1 as implied from the work of Nwogu et al. has been shown to result in a high decrease in total petroleum hydrocarbon concentration during the bioremediation of petroleum hydrocarbon contaminated soils [5]. In a distant related study on biosurfactant, a CP ratio of 16, among other factors, was shown to optimize biosurfactant production [6]. It should be noted that biosurfactant are produced by some microorganisms to aid them in degradation of hydrocarbons [7].
pH is listed among the limiting factors for soil bioremediation [8]. Remediation treatments involving alkaline pH have been shown to result in low hydrocarbon degradation efficiency [9]. In another related study, remediation of hydrocarbon contaminated groundwater has been shown to be favoured with use of reaction mixtures having acidic pH than those having alkaline pH [10]. It has been noted that acidic pH range of 4.5-5.3 favours fungal growth, while most bacteria survive better in pH range of 6.5 to 8.5 [11].
Adjustment of the pH of a contaminated environment to a value between 5.3-6.5 may thus allow for a combined effort of both fungi and bacteria in the biodegradation of the contaminants. In this study, the combine use of glycerol and a CP ratio of 16, followed by adjustment of the environmental pH to a slight acidic value of 5.5 were investigated as a means for bioremediation of petroleum hydrocarbon contaminated environment. The outcome of the study revealed the extent of the influence of combined effect of co-metabolism, phosphorus augmentation, and pH reduction on the success of bioremediation of hydrocarbon contaminated soil.
Materials and Methods
The bioremediation setup
The bioremediation setup consisted of about 5 kg soil artificially contaminated with about 500 ml of 1:1 diesel oil and used engine oil mixture in an amber coloured glass tank. Selected physicochemical properties of the uncontaminated soil and the subsequent contaminated soil which include total organic carbon (TOC), pH, nitrogen, phosphorus, moisture content, and total petroleum hydrocarbon (TPH) were determined.
About 50 ml glycerol was added to the contaminated soil in the bioremediation setup. A derived quantity of KH2PO4 was also added so as to obtain a CP ratio of 16:1. The moisture content of the contaminated soil was adjusted to about 10% using sterile warm (35°C-40°C) distilled water, and the pH was adjusted to 5.5 using 0.1 M tetraoxosulphate (VI) acid.
The moisture content was maintained between 10%-15% at weekly intervals. A control was setup alongside the bioremediation setup. The control also consisted of artificially contaminated soil. The moisture content of the contaminated soil in the control was adjusted just as in the bioremediation setup. The soils in the control and the bioremediation setup were tilled twice weekly with the aid of a disinfected hand trowel.
Monitoring of bioremediation
Soil samples were collected from the bioremediation setup and the control at weekly intervals. The samples were collected with the aid of a disinfected hand trowel, and sterile small size wide-mouth bottles of about 50 ml capacities. The samples were analysed for, Total heterotrophic bacterial (THB) population, Hydrocarbon utilizing bacterial (HUB) population, pH, and total petroleum hydrocarbon (TPH) concentration. THB and HUB were enumerated using the standard plate count method. In this method, nutrient agar (NA) plates were used for THB, while mineral salt agar (MSA) containing fluconazole were used for hydrocarbon utilizing bacteria.
Due to the insolubility of Fluconazole in water based medium, the content of a 50 mg Fluconazole capsule was used for an MSA medium volume of 300 ml so as to achieve an optimum distribution of the particles of Fluconazole in MSA plates. Petroleum hydrocarbons were supplied into inoculated MSA plates using the vapour phase transfer method, and the plates were incubated at ambient temperature (29°C-32°C) for 5-7 days. Inoculated NA plates were incubated at 37°C for 24 h.
Quantification of TPH concentration in soil samples
The TPH concentrations of the contaminated soils in the bioremediation setup and control setup were determined through spectrophotometric method. About 10 g of the soil samples were placed, separately, in a 150 ml capacity beaker, followed by the addition of 20 ml Xylene. The mixtures were agitated for about 5 min, and then filtered using a Whatman No. 1 filter paper.
The extracts from the filtration were subjected to absorbance measurement using a 721 VIS Spectrophotometer (Huanghua Faithful Instrument Co. Ltd, China) set at 420 nm. The absorbance readings of the extracts, with the aid of the equation of the straight line of the calibration graph previously obtained, were then used to calculate the TPH concentrations.
Statistical analysis
The analysis of variance (ANOVA) was used to determine if there was any significant difference between the extents of hydrocarbon degradation in the bioremediation setup and the control setup.
Results
Physicochemical properties of the soil
Selected physicochemical properties of the soil used in the bioremediation experiment are presented in Table 1. The TOC of the contaminated soil used in the experiment as determined using the Mebius method was 2.1%. Based on the TOC value and phosphorus concentration of the contaminated soil, the C/P ratio was worked out to be 1050. For the C/P ratio to be reduced to 16, a derived quantity of 35.1 g KH2PO4 was added.
| Property | Value |
|---|---|
| pH | 6.0 |
| Electrical conductivity | 18.81 mS/cm |
| Bulk density | 1.35 g.ml-1 |
| Moisture content | 0.4% |
| Porosity | 53.79 |
| TOC | 0.3% (2.1%) |
| N | (0.56%) |
| P | (0.002%) |
(*) – Values in bracket were results obtained for the contaminated soil
Table 1: Selected physicochemical properties of the soil.
Determination of the quantity of KH2PO4 to be added so as to achieve C:P ratio of 16
Addition of 50 ml glycerol to the bioremediation setup resulted in an additional carbon concentration of 0.49%. Based on the relative molecular mass and density of glycerol, 1 ml glycerol contains 0.49 g Carbon [12]. Thus 50 ml glycerol contains 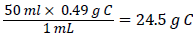 .The additional Carbon content resulting from the addition of 50 ml glycerol to the soil in the bioremediation setup is thus
.The additional Carbon content resulting from the addition of 50 ml glycerol to the soil in the bioremediation setup is thus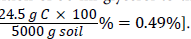 The total Carbon content in the bioremediation setup is thus 0.49%+2.1% (i.e. additional carbon content from glycerol+TOC value of contaminated soil). For C:P to be 16, P would be
The total Carbon content in the bioremediation setup is thus 0.49%+2.1% (i.e. additional carbon content from glycerol+TOC value of contaminated soil). For C:P to be 16, P would be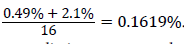 .. Thus the phosphorus content in the bioremediation setup needs to be increased by an additional amount of 0.1599%, i.e. 0.1619%-0.002% (worked out P value-P value of contaminated soil). The bioremediation setup contains 5 Kg of soil, and 0.1599% of 5 Kg is 7.995 g. There are 31 g of phosphorus in 136 g of KH2PO4. Thus 7.995 g of phosphorus would be contained in
.. Thus the phosphorus content in the bioremediation setup needs to be increased by an additional amount of 0.1599%, i.e. 0.1619%-0.002% (worked out P value-P value of contaminated soil). The bioremediation setup contains 5 Kg of soil, and 0.1599% of 5 Kg is 7.995 g. There are 31 g of phosphorus in 136 g of KH2PO4. Thus 7.995 g of phosphorus would be contained in 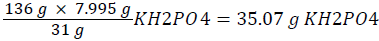 . Thus an approximate value of 35.1 g KH2PO4 was added to the contaminated soil in the bioremediation setup.
. Thus an approximate value of 35.1 g KH2PO4 was added to the contaminated soil in the bioremediation setup.
Bacterial population of the experimental setup
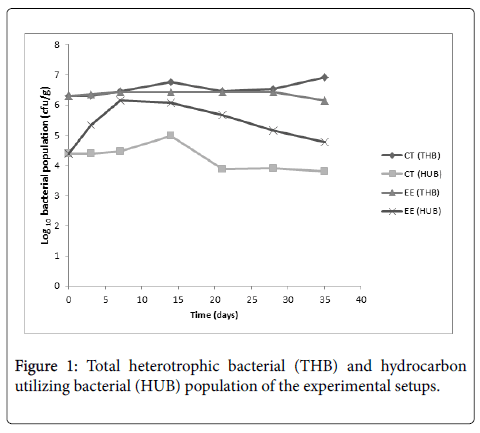
The THB population in the control ranged from 2.01 × 106 CFU.g-1 to 8.03 × 106 CFU.g-1, while the THB population in the bioremediation setup ranged from 1.43 × 106 CFU.g-1 to 2.70 × 106 CFU.g-1. The HUB population in the control ranged from 6.33 × 103 CFU.g-1 to 9.15 × 104 CFU.g-1, while the HUB population in the bioremediation setup ranged from 5.70 × 104 CFU.g-1 to 1.37 × 106 CFU.g-1.
A comparison of the bacterial population in the control and bioremediation setup is presented in Figure 1. In Figure 1 it can be seen that the bioremediation setup had a higher THB population on day 14 and day 35. Also, it can be seen that the bioremediation setup had a high HUB population than the control setup throughout the duration of the study.
Change in pH and TPH concentration
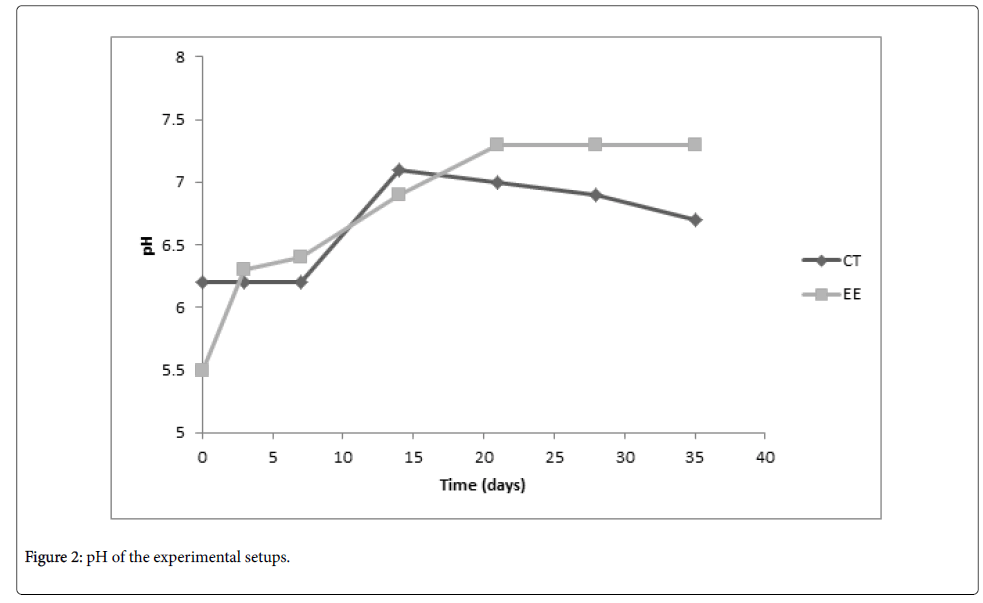
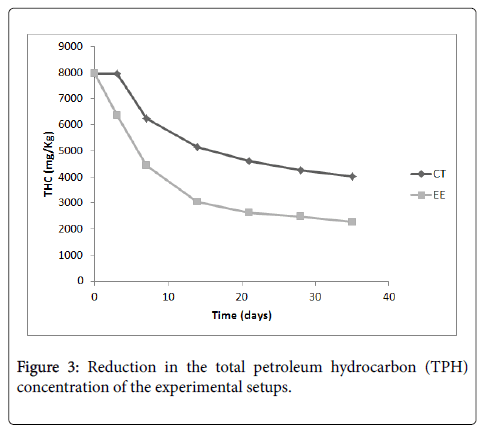
The pH and TPH concentration of the soil samples from the bioremediation setup and the control setup at weekly intervals is presented in Figure 2 and Figure 3 respectively. Figure 2 shows that in the course of the bioremediation the pH of the contaminated soil in the two setups increased from acidic values to values close to neutral pH. Figure 3 shows a general decrease in the TPH concentration, with the decrease been more in the bioremediation setup.
Statistical significance of the extent of hydrocarbon degradation
The result of the analysis of variance (ANOVA) in determining if there is a significant difference between the extent of hydrocarbon degradation in the bioremediation setup and the control setup is presented in Table 2. In Table 2 it can be seen that the Fcalculated is greater than the Ftab. (Ftabulated). There is thus a significant difference between the extents of hydrocarbon degradation in the bioremediation setup and the control setup.
| SUMMARY | ||||||
|---|---|---|---|---|---|---|
| Groups | Count | Sum | Average | Variance | ||
| CT | 3 | 12023.57 | 4007.857 | 139591.8 | ||
| EE | 3 | 6773.571 | 2257.857 | 68928.41 | ||
| Source of Variation | SS | Df | MS | Fcalculated | P-value | Ftab |
| Between Groups | 4593751 | 1 | 4593751 | 44.0605 | 0.00267 | 7.7086 |
| Within Groups | 417040.5 | 4 | 104260.1 | |||
| Total | 5010791 | 5 |
Table 2: Analysis of variance of the final TPH concentrations in the bioremediation and control setups.
Discussion
Addition of certain organic compounds which are utilizable by microorganisms to petroleum hydrocarbon contaminated soils usually enhances the attenuation of the hydrocarbons through means of cometabolism [13-15]. Glycerol, an organic compound which consist of three carbon atoms [12], is easily utilized as a carbon and energy source by many microorganisms [16-18]. The utilization of glycerol by microorganism as a co-metabolic means has been shown to lead to increase in biodegradation rate of hydrocarbons [1,2].
Apart from applying co-metabolism in the bioremediation of hydrocarbon polluted environment, the influence of other factors on the bioremediation process needs to be elucidated. Addition of glycerol and fertilizer was shown by Vasconcelos et al. to be more effective than addition of only glycerol in the removal of polycyclic hydrocarbons from hydrocarbon contaminated soil after a period of 30 days [1]. About 46% of the polycyclic hydrocarbons were removed by the combine use of glycerol and fertilizer within that period. In this study, about 72% decrease in hydrocarbon concentration was obtained (Figure 3) with the combined use of glycerol, phosphorus augmentation, and reduction of soil pH to 5.5.
The deviation between the extent of hydrocarbon reduction obtained by Vasconcelos et al. and what was obtained in this study could be attributed to the class of hydrocarbon monitored [1]. Total hydrocarbons were monitored in this study, while Vasconcelos et al. monitored a sub-class of hydrocarbons [1]. The sub-class monitored is less amenable to biodegradation compared with straight chain hydrocarbons. Thus a large extent of removal may have been observed if the total hydrocarbons were monitored. In the course of the bioremediation, the pH of the contaminated soil in the two setups increased from acidic values to values close to neutral pH (Figure 2).
From Figure 2 it can be seen that the soil already has a slight acidic pH. However, reduction of the pH of the bioremediation setup to a more acidic pH resulted in a stable value close to neutral pH, while there was a tendency in the control setup to return back to the original slight acidic pH. In some remediation studies where chemicals were used, enhanced removal of the contaminants were more effective at pH range of 3-6 [10].
This effectiveness observed at lower pH range supports the enhanced bioremediation observed at the further reduced pH used in this study. On comparing Figure 1 and Figure 2, it can be seen that in the control setup, from day 7-day 14, there was an increase in pH as well as an increase in the HUB population. The increase in the HUB population could be attributed to the improved soil moisture and tilling, which were intermittently carried out for both setups. The increase in the HUB population, which also reflects a general increase in metabolic activity, may have resulted in increasing the soil pH from an acidic value to a value close to neutral pH. In a bioremediation study in which the effect of soil pH on the biodegradation of polycyclic aromatic hydrocarbons was investigated, greatest bacterial population was observed at a soil pH of 7.5 [19]. This is in close agreement with the highest HUB population of the control setup observed at pH 7.1 in this study.
In this study, it has been revealed that the addition of glycerol, phosphorus augmentation to the tune of CP ratio of 16:1, followed by adjustment of soil pH to 5.5 resulted in about 72% decrease in hydrocarbon concentration (Figure 3). The control setup had about 50% reduction in hydrocarbon concentration. This moderate reduction in hydrocarbon concentration in the control setup could be attributed to the maintenance of soil moisture at 10%-15% and tilling, which were done for both setups. Improving soil moisture and tilling are among activities which can bring about enhanced natural attenuation of hydrocarbons in the environment [20,21].
The high reduction of hydrocarbon concentration in the bioremediation setup is supported by the relatively high population of hydrocarbon utilizing bacteria in the setup (Figure 1). Bioremediation of hydrocarbon polluted environment through means of cometabolism can thus be further enhanced by improving phosphorus content, soil moisture, reducing soil pH to a slight acidic value, and tilling. On comparing the extent of hydrocarbon reduction in the control setup and the bioremediation setup using ANOVA (Table 2), it can be seen that there is a significant difference between the extents of hydrocarbon reduction in both setups. Thus the attenuation of hydrocarbons in the bioremediation setup is significant.
Conclusion
Addition of organic compounds such as glycerol to petroleum hydrocarbon contaminated soils can enhance the attenuation of the hydrocarbons through means of co-metabolism. However, other factors such as the availability of relevant macronutrients and soil pH can influence the outcome of the bioremediation process.
In this study, the addition of glycerol, phosphorus augmentation to the tune of C:P ratio of 16:1, followed by adjustment of soil pH to 5.5 resulted in a high reduction of the total hydrocarbon concentration. The extent of hydrocarbon reduction was significant compared to a control setup where only improve moisture content and tilling were carried out.
References
- Vasconcelos U, Oliveira FJS, Franca FP (2011) Removal of high-molecular weight polycyclic aromatic hydrocarbons. Quim. Nova, 34: 218-221.
- Vasconcelos U, Oliveira FJS, Franca FP (2013) Raw glycerol as cosubstrate on the PAHs biodegradation in soil. Can J Pure Appl Sci 7: 2203-2209.
- Liebeg EW, Cutright TJ (1999) The investigation of enhanced bioremediation through the addition of macro and micro nutrients in a PAH contaminated soil. Int Biodeter Biodegr 44: 55-64.
- Brockman FJ, Payne W, Workman DJ, Soong A, Manley S, et al. (1995) Effect of gaseous nitrogen and phosphorus injection on in situ bioremediation of a trichloroethylene-contaminated site. J Hazard Mat 41: 287-298.
- Nwogu TP, Azubuike CC, Ogugbue CJ (2015) Enhanced bioremediation of soil artificially contaminated with petroleum hydrocarbons after amendment with Capra aegagrushircus (Goat) manure. Biotechnol Res Int 2015: 1-7.
- Peekate PL, Abu GO (2017) Optimizing C:N ratio, C:P ratio, and pH for biosurfactant production by Pseudomonas fluorescens. J Adv Microbiol 7: 1-14.
- Banat IM, Franzetti A, Gangolfi I, Bestetti G, Martinotti MG, et al. (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87: 427-444.
- Yerushalmi L, Rocheleau S, Cimpoia R, Sarrazin M, Sunahara G, et al. (2003) Enhanced biodegradation of petroleum hydrocarbons in contaminated soil. Bioremed J 7: 37-51.
- Wu H, Sun L, Wang H, Wang X (2016) Persulfate oxidation for the remediation of petroleum hydrocarbon-contaminated soils. Pol J Environ Studies 25: 851-857.
- Medjor WO, Akpoveta VO, Egharevba F (2017) Kinetics and physicochemical studies of surfactant enhanced remediation of hydrocarbons contaminated groundwater. Egyptian J Petrol.
- Ezeonu CS, Onwurah INE, Oje OA (2012) Comprehensive perspectives in bioremediation of crude oil contaminated environments: Introduction to enhanced oil recovery (EOR) processes and bioremediation of crude-oil contaminated sites. Croatia: InTech Pp. 143-184.
- Myers RL (2007) The 100 Most Important Chemical Compounds: A Reference Guide. Greenwood Press, Inc. Connecticut, USA.
- Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67: 503-549.
- Ubalua AO (2011) Bioremediation strategies for oil polluted marine ecosystems. Australian J Agric Eng 2: 160-168.
- Joutey NT, Bahafid W, Sayel H, Ghachtouli NE (2013) Biodegradation: Involved microorganisms and genetically engineered microorganisms: Biodegradation-Life of science. Croatia: InTech Pp. 289-320.
- Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74: 1124-1135.
- Da Silva GP, Mack M, Contiero J (2009) Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27: 30 -39.
- Suhaimi SN, Phang L-Y, Maeda T, Abd-Aziz S, Wakisaka M, et al. (2012) Bioconversion of glycerol for bioethanol production using isolated Escherichia coli SS1. Braz J Microbiol 43: 506-516.
- Pawar RM (2015) The effect of soil pH on bioremediation of polycyclic aromatic hydrocarbons (PAHS). J Bioremed Biodeg 6: 291.
- Wegwu MO, Uwakwe AA, Anabi MA (2010) Efficacy of enhanced natural attenuation (land farming) technique in the remediation of crude oil-polluted agricultural land. Arch Appl Sci Res 2: 431-442.
- Chikere CB, Azubuike CC, Fubara EM (2017) Shift in microbial group during remediation by enhanced natural attenuation (RENA) of a crude oil-impacted soil: a case study of Ikarama Community, Bayelsa, Nigeria. 3 Biotech 7: 152.
Copyright: © 2018 Peekate LP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.