Journal of Bone Research
Open Access
ISSN: 2572-4916
ISSN: 2572-4916
Case Report - (2022)Volume 10, Issue 4
Grisel's syndrome is defined as non-traumatic subluxation of the atlantoaxial joint, following an inflammatory process in the upper respiratory tract. Patients with Down syndrome have higher risks of developing atlantoaxial instability. Accompaniment of Grisel’s syndrome and Down syndrome was not perused in recent investigations. To our knowledge, only one case of Grisel’s syndrome in an adult patient with Down syndrome has been reported. In this study, we are presenting a case of Grisel syndrome in a 7-year-old boy with Down syndrome, following lymphadenitis. A 7-year-old boy with Down syndrome was admitted in orthopedic ward of shariati hospital with possible diagnose of Grisel’s syndrome and treated him with a mento-occipital traction for 10 days. In this case report for the first time, we represent a pediatric who suffered Down syndrome with Grisel’s syndrome. We also imitated a simple and applicable non-surgical treatment for Grisel’s syndrome.
Grisel’s syndrome; Down syndrome; Atlantoaxial joint
Grisel's syndrome is defined as non-traumatic subluxation of the atlantoaxial joint, following an inflammatory process in the upper respiratory tract. Grisel's syndrome is related to Pharyngitis, Nasopharyngitis, Adenotonsilitis, Tonsillar abscess, Parotitis, Cervical abscess, Otitis media [1]. This is a rare syndrome that mostly occurs among the pediatric population [1]. Different hypotheses were proposed for Grisel syndrome pathology like the Presence of laxity in the cervical ligament which can be seen in the childhood population. Moreover, spasms of cervical muscles because of the inflammatory process [2]. Patients with Down syndrome have higher risks of developing atlantoaxial instability, and atlantoaxial instability has been reported in up to 27% of the Down syndrome population [3]. Ligamentous laxity and skeletal anomalies in the cervical region such as Os odontoideum has been suggested as the predisposing factors for atlantoaxial instability in Down syndrome patients [4]. Accompaniment of Grisel’s syndrome and Down syndrome was not perused in recent investigations. To our knowledge, only one case of Grisel’s syndrome in an adult patient with Down syndrome has been reported. In this study, we are presenting a case of Grisel syndrome in a 7-year-old boy with Down syndrome, following lymphadenitis [5,6].
A 7-year-old boy with Down syndrome was referred to our clinic with torticollis and neck deviation from 3 weeks prior to visit (Figure 1). He had suffered from a cervical lymphadenitis four weeks earlier. First He had been treated with Ibuprofen (10 mg per kg per dose every 8 hours) and Biperidin (2 mg every 12 hours) with the diagnosis of acute dystonia by a neurologist in an outpatient setting, but the symptoms did not improve.
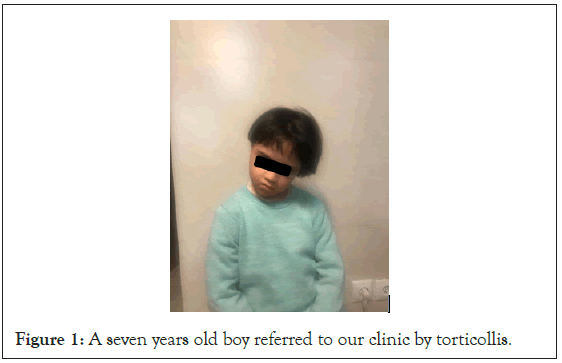
Figure 1: A seven years old boy referred to our clinic by torticollis.
Then he had been admitted to hospital in the service of infectious diseases with the diagnosis of acute lymphadenitis and received IV antibiotics (Clindamycin 150 mg every 6 hours). During the same time, he had been treated with cervical orthosis for 1 week and following by cervico-thoracic orthosis for 2 weeks. As the symptoms had persisted, he was referred to orthopedics clinic. Cervical CT scans and radiographs were obtained, which showed signs of C1- C2 subluxation (Figure 2).
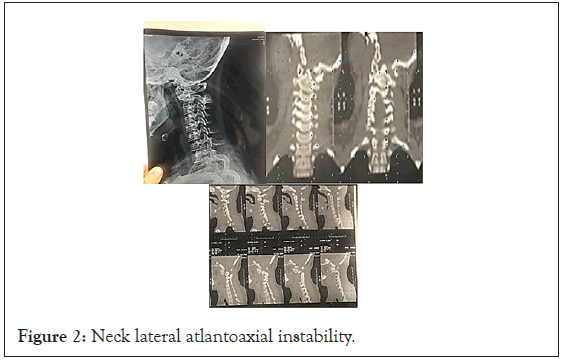
Figure 2: Neck lateral atlantoaxial instability.
The patient was admitted in orthopedic ward of Shariati hospital with possible diagnose of Grisel’s syndrome and treated him with a mento-occipital traction for 10 days. We followed Ciftdemir, et al. [7], protocols for mento-occipital halter traction. The patient’s characteristics such as age and weight determine the weights used for mento-occipital traction: 1 kg weights are used for patients younger than 10 years of age and weighing less than 25 kg, 2 kg weights are used for patients older than 10 years and weighing more than 25 kg. Our patient was examined twice daily and neurological condition, neck pain, and range of motion were examined and recorded twice daily.
After 2 weeks patient’s symptoms vanishing away completely. He did not suffer from neck pain and torticollis anymore. Rotatory motions of the neck were in the normal range and painless. Also, radiologic findings showed significant healing. We also follow our patient every 2 weeks for 2 months and after 6 months. In our follow-ups, our patient did not have any sign or symptom of atlanto-axial rotatory instability. We also evaluated our patient in the sixth month by spiral CT scan which shows normal range ADI and no evidence of subluxation (Figure 3).
Figure 3: Patient neck appearance 6 months after treatment. No torticollis or neck pain and full neck range of motion.
In this article we reported a child with trisomy 21 who suffered from Grisel’s syndrome and was treated with a non-operative traction with acceptable result. As far as we know this is the first time Grisel’s syndrome was reported in a child with trisomy 21.
Atlantoaxial instability
Atlantoaxial Instability (AAI) characterize by the excessive motion of C1 relative to C2, often defined in children by an Atlanto-Dental Interval (ADI) of greater than 4.5 mm [1]. Only in the position of neutral the inferior aspect of the C1 articular process in full association with the corresponding part of C2. The predominant stabilizer of atlanto-axial joint is the transverse ligament. Furthermore, Alar ligaments have a crucial role in holding stability [1]. Management of AAI is based on the amount of ADI. According to recent studies: If ADI is lower than 4.5 mm, treatment will be conservative and there will be no restriction of activities necessary. If ADI is 4.5 to 10 mm depend on the presence of neurologic symptoms treatment will be high-risk activities limitation or C1-C2 fusion. If ADI is greater than 10 mm, treatment will be posterior fusion and instrumentation [2,8] (Table 1).
| ADI | Treatment |
|---|---|
| <4.5 mm | Conservative treatment No restriction of activities |
| 4.5-10 mm | Depending on the presence of neurologic symptoms Limitation of high-risk activities Or C1-C2 fusion |
| >10 mm | Posterior fusion and instrumentation |
Table 1: AAI management according to ADI.
Grisel’s syndrome
Atlantoaxial Subluxation (AAS), following an inflammatory process (e.g. infection or post-surgery conditions) in the head and neck region, is called Grisel's syndrome. A few explanations have been proposed for the development of Grisel's syndrome [9]. The process starts with inflammation in the pharyngeal tissue. Then, the exudate extends to the craniovertebral junction hematogenously, via the direct connection between the pharyngovertebral veins and the periodontoidal venous plexus. Since there is no lymph node present around this plexus, the exudate could directly extend to the C1-C2 joint. The infectious exudate damages the surrounding tissues. The exudate, also, triggers the spasm of the cervical muscles. On the other hand, hyperemia following the inflammation causes the abnormal loosening of the transverse ligament [10]. Altogether, the direct chemical damage, the abnormal spasm of the cervical muscles, and the loosening of transverse ligament contribute to the occurrence of AAS [2,10,11]. Several theories attempt to explain the exact physiopathology of C1-C2 subluxation and instability in Grisel’s syndrome. One theory is that Grisel’s syndrome happens due to the body's endeavor to decompress the inflamed tissue by spasm and consequent subluxation [11].
In other theory hyperemia state as a result of inflammation or infection was the main reason for this pathology. They hypothesized that hyperemia will cause decalcification of T1 and T2 and also is the root of loosening of the transverse ligament and other ligaments which have a role in atlantoaxial stability [12]. Results about ligamentous laxity due to inflammation are controversial [13,14]. Moreover, pharyngovertebral veins have a significant role in Grisel’s syndrome pathology, by transitions of pus from the upper respiratory tract to the paravertebral area [10]. One important theory is the two-hit hypothesis. In this hypothesis, prior cervical ligaments laxity which is more common in children is the first hit. The second hit is the effect of inflammatory mediators which were transferred to the paravertebral area by pharyngovertebral vein plexus. These mediators cause cervical spasms and consequent subluxation [3].
According to fielding classification, there are four types of nontraumatic atlantoaxial rotatory subluxation. In type 1 there is no anterior dislocation and the only atlas is rotated on the odontoid. In type 2, we have rotation of atlas on one particular process and 3-5 mm anterior dislocation. In type 3, anterior displacement is more than 5 mm. type 4 is defined when we have rotatory fixation and posterior displacement of Atlas. Usually, types 3 and 4 cause spinal cord compression and neurological symptoms [15] (Table 2).
| Type 1 | No anterior dislocation, only atlas is rotated on odontoid |
| Type 2 | We have rotation of atlas on one particular process and 3-5 mm anterior dislocation |
| Type 3 | >5mm anterior dislocation |
| Type 4 | Rotatory fixation and posterior displacement of Atlas |
Table 2: Fielding classification.
Trisomy 21 and atlantoaxial instability
Spontaneous atlantoaxial subluxation occurs in 10 to 30 percent of trisomy 21 patients. Only 1 to 2 percent of patients are symptomatic [16,17]. The congenital skeletal anomalies such as Os odontoideum, and pre-existing ligamentous laxity in the Down syndrome population may put the patients with Down syndrome, like our patient, at higher risks for developing Grisel’s syndrome after an infectious process [4]. Furthermore, in trisomy 21 patients there is a possibility for malformation, hypoplasia or complete absence of odontoid process which can be a predisposing factor for atlantoaxial instability [18]. Various studies suggested serial examination and radiologic follow ups (lateral neck radiology in flexion and hyper-extension positions) for trisomy 21 patients at least twice between the age of 5 to 15 [19]. On the other hand, Hengartner et al in their study indicated that routine radiologic follow up in Down syndrome patients is not beneficial for detection of atlantoaxial instability [4].
Grisel’s syndrome in children
Yamazaki et al. reported the occurrence of AAS following a retropharyngeal infection in a 26-year-old male with Down syndrome. The patient presented with neck pain for two months and gait disturbance. He was treated with IV antibiotics and his cervical spine was immobilized with Halo-Vest for three months. However, after the removal of the Halo-Vest, the symptoms returned. The recurrence of AAS necessitated surgical treatment. Yamazaki and his colleagues believed that the ligamentous laxity and presence of Os odontoideum contributed to the failure of conservative therapy [9]. There was not any study that represents Grisel’s syndrome in a child who suffers from Down syndrome. In our study patient responded to conservative therapy. In this patient, we demonstrated that adequate and proper conservative treatment can be advantageous for pediatric who suffer from Grisel’s syndrome accompany by Down syndrome [20-22].
We are suggesting that the possibility of AAS after an infectious process needs special clinical attention in patients with Down syndrome. Early diagnosis and start of treatment at the right moment could prevent the development of myelopathy, and the need for surgical intervention. We are suggesting that the Down syndrome patients diagnosed with an infection in the pharyngeal region may benefit from assessment for AAS and follow-up imaging with cervical spine series. On the other hand, the ligamentous laxity in the Down syndrome population can contribute to a more severe presentation of the disease, and increased risk of developing myelopathy in comparison with Grisel’s syndrome in patient without trisomy 21.
In this case report for the first time, we represent a pediatric who suffered Down syndrome with Grisel’s syndrome. Several studies indicated Down syndrome will increase the risk of Grisel’s syndrome after any type of upper respiratory tract inflammation. As a result, after an upper respiratory tract or gastrointestinal infection or inflammation, there will be a high risk for the presentation of Grisel's syndrome in patients with Down syndrome. Close followup with X-ray or CT scan will be valuable for early diagnosis of Grisel's syndrome in Down syndrome patients who were suffered from infection.
In this study, we also imitated a simple and applicable non-surgical treatment for Grisel’s syndrome. Contrary to the tendency for surgical treatment of Grisel’s syndrome, our case completely responded to an on-time conservative treatment. Consequently, conservative treatment in Grisel’s syndrome may have acceptable results and it is important to be aware about these kinds of treatments.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Moghadam RS, Violas P, Mehrpour SR, Nabian MH (2022) Grisel's Syndrome and Down Syndrome: A Case Report. J Bone Res. 10:170.
Received: 05-May-2022, Manuscript No. BMRJ-22-17760; Editor assigned: 09-May-2022, Pre QC No. BMRJ-22-17760 (PQ); Reviewed: 23-May-2022, QC No. BMRJ-22-17760; Revised: 30-May-2022, Manuscript No. BMRJ-22-17760 (R); Published: 07-Jun-2022 , DOI: 10.35248/2572-4916.22.10.170
Copyright: © 2022 Moghadam RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.