Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Mini Review - (2022)Volume 13, Issue 2
Hypertension and breast cancer are two common diseases occurring in women. Clinical studies have shown increased breast cancer incidence in hypertensive women. Several lines of evidence demonstrate that G proteincoupled Receptor Kinase 4 (GRK4) could be a common risk factor for hypertension and breast cancer. This article reviews our current understanding of molecular mechanisms of GRK4 in hypertension and breast cancer.
G protein-coupled receptor kinase 4 (GRK4), hypertension, breast cancer
Hypertension and breast cancer are two common diseases in women worldwide. Of all adult women, 1 in 3 have hypertension and 1 in 8 will develop breast cancer during her lifetime. Prevalence of both diseases increases with age. After menopause, hypertension prevalence increases to 75%. About 50% of breast cancer patients are in this age group. A recent meta-analysis of 30 studies with 11,643 cases of breast cancer has shown a statistically significant association between hypertension and increased breast cancer risk (RR: 1.15; 95% CI: 1.08-1.22) [1]. The fact that breast cancer incidence is 13%-15% higher in hypertensive women after adjustment of known common risk factors indicates an intrinsic linkage between these two pathological conditions. Our recent studies suggest that G protein-coupled receptor kinase 4 (GRK4) could be a molecule underlying pathological mechanism of hypertension and breast cancer.
GRK4
G Protein-Coupled Receptor Kinases (GRKs) constitute a family of seven serine/threonine protein kinases that specifically recognize and phosphorylate agonist-activated G Protein-Coupled Receptors (GPCRs). GRK mediated receptor phosphorylation is one of the well-characterized mechanisms for GPCR desensitization (Figure 1) [2]. GRK family members can be subdivided into three main groups based on sequence homology: rhodopsin kinase or visual GRK subfamily (GRK1 and GRK7), the β-adrenergic receptor kinases subfamily (GRK2/GRK3) and the GRK4 subfamily (GRK4, GRK5 and GRK6). These kinases share certain characteristics but are distinct enzymes with specific regulatory properties. GRK2, 3, 5 and 6 are ubiquitously expressed in mammalian tissues, whereas GRK1, 4 and 7 are confined to specific organs. GRK1 and 7 are expressed in retinal rods and cones, respectively, and GRK4 is present in testis, brain and kidney [3-6].
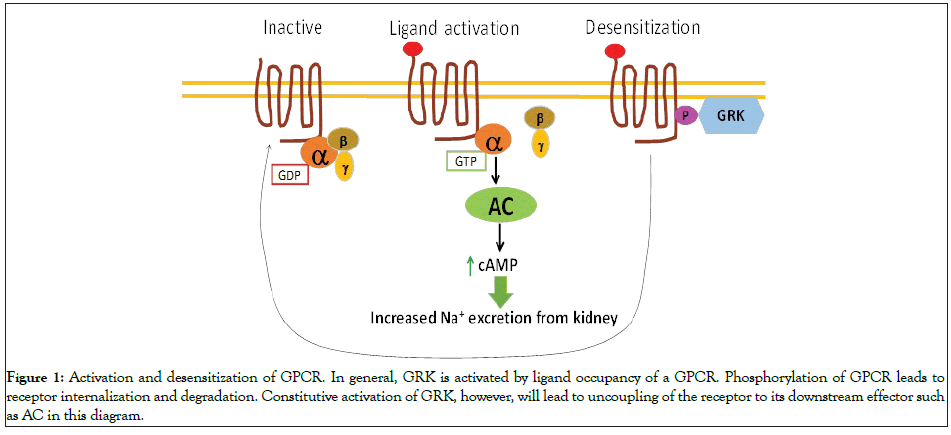
Figure 1: Activation and desensitization of GPCR. In general, GRK is activated by ligand occupancy of a GPCR. Phosphorylation of GPCR leads to receptor internalization and degradation. Constitutive activation of GRK, however, will lead to uncoupling of the receptor to its downstream effector such as AC in this diagram.
GRK4 is encoded by a single gene of 16 exons. The GRK4 gene transcript undergoes extensive alternative splicing to generate four distinct forms of GRK4 mRNA that encode four isoforms of the GRK4 protein, , β, γ and δ.The α isoform is encoded by the full length gene transcript. The remainder of the isoforms arise from GRK4 mRNAs missing either exon 2 (α) or exon 15 (β) or both (γ) [3].
GRK4 and hypertension
Hypertension is a complex trait determined by both genetic and environmental factors and is a major public health problem due to its high prevalence and concomitant increase in the risk for cardiovascular disease. Essential hypertension and salt sensitivity (>10% change in mean arterial pressure following a change in sodium intake from high to low, or vice versa) are two closely related but independent conditions that are influenced by multiple interacting factors. Among environmental factors, dietary salt intake is the most common and important risk factor for hypertension [7]. The kidney is a key blood pressure regulatory organ acting by regulating electrolytes and water homeostasis. About 75% of sodium excretion is regulated in the proximal tubule [8]. Regulation of sodium excretion from renal proximal tubules is mediated by a GPCR, the type one Dopaminergic Receptor (D1R), and corresponding GRK, GRK4 [8-10]. Under normal circumstances, high salt intake activates D1R which increases sodium excretion mediated by second messenger cyclic AMP (cAMP). The major function of GRKs is to mediate signaling termination through phosphorylation of activated G Protein- Coupled Receptors (GPCRs). Phosphorylated GPCRs recruit arrestins which bind to the receptors with high affinity and bring the receptor to clathrin pits for recycling or degradation. This is a necessary process for termination of G-protein
The natriuretic function of D1R relies on coupling of activated D1R with G-protein. Defective coupling of D1R to the adenylate cyclase has been found in prehypertensive Dahl salt-sensitive rats [12] and in Spontaneously Hypertensive Rats (SHR) [13]. Compared with normotensive individuals, responses of kidney proximal cells to the D1R agonist, fenoldopam, was blunted in hypertensive patients [14]. All these studies demonstrated the relationship between abnormalities in renal D1R signaling and hypertension.
What is the cause of the functional defect of D1R in the kidney? Earlier studies suggested that attenuated response of kidney proximal tubule cells from hypertensive patients was a result of enhanced phosphorylation of D1R [4, 14]. GRK4 SNPs is one mechanism responsible for enhanced kinase activity. Our previous studies have identified three SNPs, R65L (rs2960306), A142V (rs1024323), and A486V (rs1801058) all of which account for higher GRK4 activity and uncoupling between the D1R and its downstream effector [4,15]. It should be mentioned that unlike wild type GRK4, these variants have increased basal activity which causes enhanced D1R phosphorylation and functional attenuation. The causal relationship between GRK4 and hypertension was confirmed in a transgenic mouse model [4]. The transgenic mice expressing GRK4γ A142V were hypertensive whereas those expressing the wild-type GRK4γ were normotensive. Furthermore, intravenous infusion of fenoldopam (specific D1R agonist) increased urine flow and sodium excretion in GRK4γ wild-type mice but not in the hypertensive GRK4γ A142V transgenic mice [4].
GRK4 and breast cancer
The potential link between GRK4 and breast cancer has been supported by several lines of evidence from clinical and experimental studies. As mentioned above, GRK4 expression is restricted to the testis, brain and kidney. However, high levels of GRK4 protein are found in invasive breast cancer tissues but not in the normal surrounding breast tissues [16]. We have tested 7 breast cancer cell lines and found that GRK4 protein was expressed in all cancer lines but not in benign mammary epithelial cells. The α isoform is the predominant type of GRK4 in breast cancer cells [17].
The function of GRK4 in breast cancer cells is to stimulate proliferation rather than inhibition of apoptosis. This has been confirmed by the experiments of siRNA silencing and GRK4 over-expression [17]. Whether or not the proliferation promoting function of GRK4 is mediated by the canonical GPCR pathway is still unclear. Matsubayashi, et al. have shown that overexpression of GRK4 isoforms in MCF-7 cells increased cell proliferation via activation of the ERK1/2 and JNK MAPK pathways [16]. The activation of ERK1/2 and JNK MAPK requires β-arrestin suggesting that a delayed GPCR reaction is involved [18].
Under normal physiological circumstances, GKR4 is activated only by a ligand occupied GPCR resulting in internalization of activated receptor and termination of the signal transmitted (Figure 1). Previous studies of ours and others have shown that the variants of GRK4 with SNPs have increased activity that caused uncoupling between D1R and its effector in the kidney and hypertension [4,14]. Those hypertension-related GRK4 SNPs were also found in breast cancer cell lines and breast cancer tissues. Of seven breast cancer cell lines tested, six contain at least one SNP. A142V (rs1024323) is the most frequently occurred SNP [17]. Using global Differential Allele- Specific Expression (DASE) analysis, Gao, et al. has identified 60 breast cancer risk loci in the mammary epithelial cells derived from breast cancer patients. Notably, among them two GRK4 variants, R65L (rs2960306) and A142V (rs1024323) increased breast cancer risk by 3.01 and 2.37 fold respectively [19]. These data suggest that in addition to overexpression, imbalanced expression of GRK4 variants could be a risk factor for tumorigenesis.
Abnormal expression may also account for enhanced activity of GRK4 in breast cancer. We have shown before that GRK4 expression in renal proximal tubule cells is regulated by cMyc [20]. cMyc is an oncogene that is upregulated or amplified in breast cancer and other types of cancer [21, 22]. Knocking down cMyc in MCF-7 cells significantly slowed down the growth rate (2.6-fold increase in doubling time of these cells). Re-expression of GRK4 resumed the growth rate nearly to the level of wild type MCF-7. The effect of the GRK4γ variant with three SNPs is more potent than its non-SNP counterpart [17]. These results indicate that GRK4 is a newly identified downstream effector of cMyc for cell proliferation and GRK4 variants are more active which is consistent with the finding that GRK4 variants are associated with increased risk of breast cancer [19].
Targeting GRK4 as a novel means to treat breast cancer patients especially those with hypertension
Since a majority of breast cancer is invasive at diagnosis, systemic treatment or so-called adjuvant therapy, is a necessary component of a therapeutic plan to prevent distant metastasis. Choice of therapeutic agents is based on the type of breast cancer. In general, breast cancer can be divided into three types determined by their expression of Estrogen Receptor (ERα), Progesterone Receptor (PR), and HER2. Hormonal therapy using antiestrogens (e.g., tamoxifen) [23] or aromatase inhibitors (e.g., letrozole) [24] has greater benefit in hormone receptor positive cancer patients which constitutes 70- 75% of breast cancer cases. Another category of breast cancer are the ones that express HER2. HER2+ breast cancer can be treated with monoclonal antibody against HER2 (Herceptin) or Herceptin conjugated chemotherapy agent (TD-M1) [25]. Both hormonal and anti-HER2 therapies are targeted therapy with relatively fewer side effects compared with non-selective chemotherapy. The third type of breast cancer is called Triple-Negative Breast Cancers (TNBC) that don’t have estrogen or progesterone receptors and also don’t overexpress HER2 protein. Because the cancer cells lack these proteins, treatment options for triple-negative breast cancer are limited. Non-specific chemotherapy is the main systemic treatment option for TNBC [26,27]. While only about 10% of breast cancers are triple-negative, this type of breast cancer is considered to be more aggressive and have a poorer prognosis than other types of breast cancer. Finding a molecule for targeted therapy of such an aggressive type of breast cancer is still in urgent need. GRK4 could be a good candidate.
Ideal candidates for the druggable genome have the characteristics of increased expression and/or activity of the gene in the disease state combined with the lack of phenotype with the knockout of the gene. GRK4 meets all of the characteristics of an ideal drug target with the additional characteristic of possible reversion of two of the costliest diseases in medicine. There is clear data showing that targeting GRK4 by anti-sense oligodeoxynucleotides in Spontaneous Hypertension Rats (SHR) led to reversion of D1R uncoupling and hypertension [27]. And growth of breast cancer cells can be inhibited in vitro by GRK4 silencing [17]. Targeting GRK4 is likely to be translated to precision medicine for the care of hypertension and breast cancer patients. This will rely on development of molecular diagnosis and specific inhibitor of GRK4.
GRK4 is an important regulator of sodium excretion in renal proximal tubule cells. Malfunction of GRK4 leads to hypertension. GRK4 has been found in breast cancer cells with the function of proliferation promotion. These findings indicate that GRK4 is a common risk factor for both hypertension and breast cancer. Therefore, GRK4 could be a therapeutic target for breast cancer and hypertension especially for the patients with both diseases.
This work was supported by the National Heart Lung and Blood Institute HL074940, and National Institute of Diabetes Digestive Diseases and Kidney DK039308.
The authors have nothing to disclose.
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
Citation: Yue W, Gildea JJ, Xu P, Felder RA (2022) GRK4, A Potential Link between Hypertension and Breast Cancer. J Cell Sci Therapy. 13:343.
Received: 15-Feb-2022, Manuscript No. JCEST-22-15870 ; Editor assigned: 17-Feb-2022, Pre QC No. JCEST-22-15870 (PQ); Reviewed: 01-Mar-2022, QC No. JCEST-22-15870 ; Revised: 08-Mar-2022, Manuscript No. JCEST-22-15870 (R); Published: 15-Mar-2022 , DOI: 10.35248/2157-7013-22.13.343
Copyright: © 2022 Yue W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.