Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2022)Volume 10, Issue 7
Aim: We hypothesise that Bifidobacterium bifidum LMG 11041 and Bifidobacterium longum LMG 13197 may inhibit the survival and growth of Cronobacter sakazakii in Reconstituted Infant Milk Formula (RIMF) due to their probiotic properties. The main objective of the study is to determine whether the Bifidobacterium bifidum LMG 11041 inhibits the growth and survival of the enteropathogen Cronobacter sakazakii in RIMF.
Objectives: The specific objectives were to screen commercial infant formula for the presence of Cronobacter sakazakii; to determine the change in levels of Cronobacter sakazakii and Bifidobacterium species in RIMF over a specified period at different temperatures; to determine how the pH of RIMF is affected by the presence of probiotics and pathogens.
Methods: Neonatal bacterial sepsis is one of the leading causes of infant mortality. Among many of the organisms responsible for neonatal sepsis is Cronobacter sakazakii. It is especially dangerous as it has been found to proliferate in powdered Reconstituted Infant Milk Formula (RIMF). The problem is due to prolonged time between reconstitution and administration, allowing the pathogen to multiply to infectious levels. This study determined whether the inclusion of Bifidobacteria species in infant formula can limit the growth and survival of C. sakazakii after reconstitution of the milk. A 1:1 ratio of a two-strain cocktail of C. sakazakii isolated from RIMF, B. bifidum LMG 11041 and B. longum 13197 was inoculated into RIMF and incubated at different temperatures after which viable numbers of bacteria were determined.
Results: There was no difference in the growth and survival of C. sakazakii in the presence and absence of B. bifidum at all temperatures tested. The numbers of bifidobacteria did however increase. A considerable drop in pH occurred in RIMF inoculated with B. bifidum and C. sakazakii. This effect was not observed when the RIMF was inoculated with C. sakazakii alone. There was no statistically relevant interaction between the numbers of C. sakazakii when incubated in the presence or absence of Bifidobacteria species at the 10% mean difference threshold.
Conclusion: The presence of B. bifidum LMG 11041 or B. longum LMG 13197 in RIMF did not have a statistically relevant inhibitory effect on the survival of C. sakazakii.
Cronobacter sakazakii; Bifidobacterium bifidum; Probiotics; Infants; Infection
The study of probiotics is an emerging area in the field of microbiology that encompasses all organisms that, according to the World Health Organization (WHO), confer a health benefit to the host when applied in sufficient amounts. These beneficial bacteria are naturally found in the gastrointestinal tract where their role is thought to be most pronounced. They also play pivotal roles in the manufacturing of a range of different everyday food products including cheese, bread, yoghurt, beer and wine [1]. The range of benefits associated with the use of probiotics includes lowered blood pressure, lowered cholesterol, improvement of immune functioning, aided digestion of fibre and thereby stimulation of bowel movement and neutralization of carcinogenic compounds in the gut [2-4]. They are most commonly used to prevent diarrhoea due to secondary infection by pathogenic intestinal organisms such as C. difficile [5] which normally prevented from causing infection by these probiotic organisms either by inhibition, competitive exclusion or by some inhibitory factor [6,7].
Of these probiotic cultures, Bifidobacterium species are non-motile, gram-positive, fastidious, anaerobes that have been demonstrated to inhibit certain pathogenic bacteria [8,9]. They are some of the first colonizers of the infant gut [10], where they are thought to play a role in protection against pathogenic species as well as aiding in the establishment of a stable, dynamic gut environment [8].
It is known that Bifidobacterium species constitutes approximately 90% of the gut microbiota of infants that are born by natural delivery, where they are received from the mother through ingestion of fluids in the birth canal [10]. The figure for infants delivered by cesarean section is significantly lower [11]. A common denominator between neonates that suffer from C. sakazakii infection is that most of them are delivered by caesarean section and therefore did not receive the normal microbiota that a child that passes through the birth canal during natural delivery would [8]. The formula-fed infants are generally more susceptible to neonatal infectious diseases such as meningitis. Neonatal meningitis is a cause of infection by Cronobacter sakazakii (formerly Enterobacter sakazakii).
Cronobacter sakazakii is a gram-negative, non-spore-forming straight rod of the Enterobacteriaceae family that has a mortality rate of between 40%-80% in infected neonates [8]. The organism can be found on food products where it poses a threat mainly to the immunocompromised i.e., children and the elderly. It has a very low infective dose of approximately 3 cfu’s/ml which is one of the reasons this pathogen should not be viewed lightly [12].
Breastfeeding is an important avenue for the transfer of probiotic organisms [13]. Most infants that suffered from C. sakazakii infection were fed reconstituted powdered infant formula [14].
No research has been done using Bifidobacteria species to inhibit C. sakazakii in reconstituted powdered infant milk formula and most of the experiments designed to date focused on using human cell lines and mucus to test for exclusion of enteropathogens by different probiotic organisms. This experiment on the effect of B. bifidum on the growth and survival of C. sakazakii in RIMF will contribute to the understanding concerning this area of the field, specifically since it is thought that C. sakazakii proliferates in the milk as soon as reconstitution takes place to levels above its infectious dose.
Evidence has suggested that the incorporation of probiotic organisms into the powdered infant milk formula affects the growth and survival characteristics of the opportunistic enteropathogen Enterobacter sakazakii [15]. Their effect on the viable numbers of C. sakazakii is necessary to prove or disprove the theory that they, being probiotic organisms like L. bulgaricus which is known to inhibit enteropathogens including E. sakazakii [16] have the same ability and could prove a possible avenue for the decrease in several cases, severity, duration or total elimination of the problem of neonatal meningitis caused by C. sakazakii.
Prophylactic administration and supplementation, possibly through the powdered infant feed, could protect the neonates from pathogenic organisms in reconstituted infant milk formula (FAO/WHO, 2004).
Preparation of infant formula
The powdered infant formula was reconstituted according to the manufacturer’s specifications as stated on the container. The chosen formula did not contain probiotic cultures as none was specified on the packaging label.
Micro-organisms
B. bifidum LMG11041 and B. longum 13197 were obtained from the Belgian culture collection and 2 strains of C. sakazakii, (strain designated P1 and PM1) were obtained from the Department of Food Sciences, Stellenbosch University. These strains were originally isolated from powdered infant formula.
Maintenance of cultures
C. sakazakii was maintained in nutrient broth with 20% glycerol and kept at -20°C. B. bifidum and B. longum were kept in MRS-cys-HCL broth with 20% glycerol at -20°C.
Resuscitation of cultures and preparation of inocula
An aliquot of the frozen culture (100 µl) was inoculated in 1 ml nutrient broth and MRS agar supplemented with 0.05 v/v cysteine-HCL for C. sakazakii and B. bifidum respectively. The MRS-cys-HCL broth was incubated anaerobically with anaerocult A gaspaks in anaerobic jars at 37˚C while the nutrient broth was incubated aerobically, both at 37°C for 48 hr. For the preparation of the inocula, a loop full of each of the C. sakazakii strains was used to inoculate 20 ml of nutrient broth. The same was done for B. bifidum, using 20 ml of MRS-cys-HCl (0.05% v/v) broth. The cultures were then incubated under appropriate conditions as was done in resuscitation.
Screening of infant formula for C. sakazakii
10 ml of the reconstituted commercial infant formula was added to 90 ml of sterile water at room temperature and mixed thoroughly. 10 ml of this suspension was then added to 90 ml of Enterobacteriaceae Enrichment (EE) broth and left overnight at Room Temperature (RT). 1 ml of this suspension was then plated onto Chromocult™ Enterobacter sakazakii agar (Merck). The plates were incubated for 24 hr at 37°C.
Inoculation of reconstituted milk formula
The broth cultures were harvested by centrifuging at 13400 rpm for 15 minutes. The pellets were then re-suspended in quarter strength Ringers solution. The original concentration, of bacteria for both pathogen and probiotic was adjusted to a 0.5 McFarland standard, which corresponds to 1.5 × 108 CFU/ml.
In addition to this, the absorbance of the cultures was adjusted to an absorbance of 0.362 at 600 nm wavelength. A 1:1 ratio of pathogen: probiotic was used for inoculation. 1 ml each for C. sakazakii two strain cocktail and B. bifidum LMG11041 was added to 98 ml of RIMF. 1 ml of activated C. sakazakii was added to another 99 ml of RIMF as control. The inoculated milk was then incubated at 4°C, RT and 37°C for 8 hr. 1 ml samples were taken from the bottles at 0,2,4,6 and 8 hrs of incubation for viable count analysis and pH measurements. The experiment was repeated three times on separate days.
Enumeration of bacteria
The samples were serially diluted in quarter-strength Ringer’s solution at RT. 100 µl of the appropriate dilutions were plated out in triplicates on VRBG agar for C. sakazakii and MRS-cys-HCL agar for B. bifidum. The plates were incubated.
Measurement of pH
The pH of the subsamples of the experiment and control incubated at RT and 37°C were measured each of the sampling times. The pH was measured using a Crison BASIC 20 pH meter.
Statistical analysis
The averages of numbers of organisms growing on the differential media were converted to log CFU/ml and line graphs of the different variables, namely the number of viable organisms and pH at different temperatures were constructed over time from the statistically relevant data recorded in the tables. The log CFU/ml of C. sakazakii and B. bifidum were superimposed and grouped on the appropriate graph according to temperature. The pH at 37°C and RT were represented on a different graph.
Statistical analysis using SAS v8.1 was done to determine the level of significance of the data set based on the consistency of the set points. A 5% difference was used as the threshold for being considered the same and a difference of up to 10% was tolerated.
Only statistically relevant plate counts were converted to averages and used in the analysis.
There were no typical C. sakazakii colonies (turquoise) on the chromocult medium after incubation. This indicated that the infant formula did not harbour this pathogen. The uncontaminated RIMF was then used subsequently in experiments, where it was spiked with the C. sakazakii test strains (Figures 1-3).
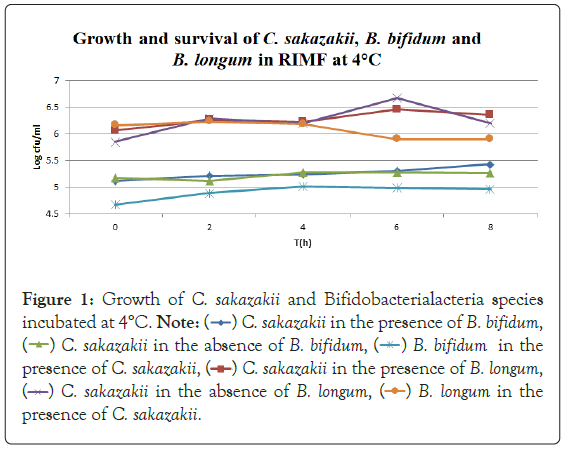
Figure 1: Growth of C. sakazakii and Bifidobacterialacteria species
incubated at 4°C.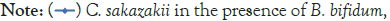
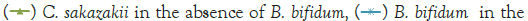
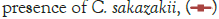
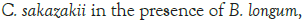
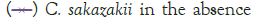
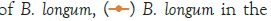
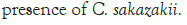
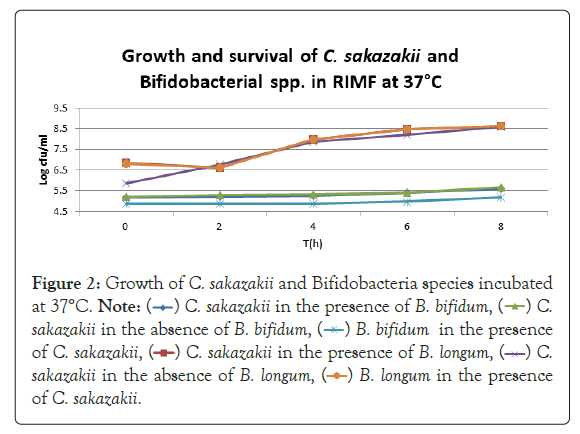
Figure 2: Growth of C. sakazakii and Bifidobacteria species incubated
at 37°C.
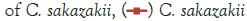
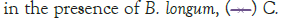
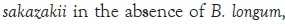
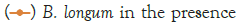
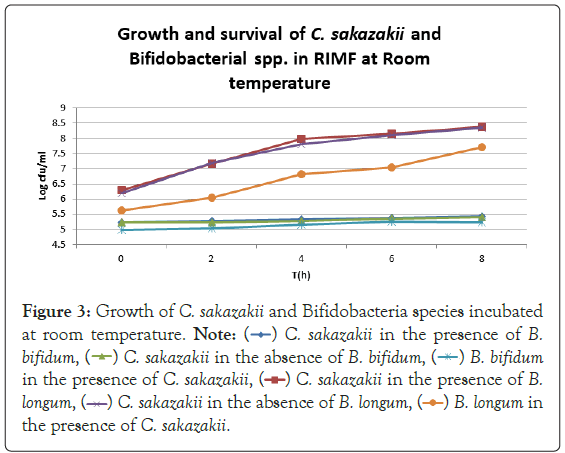
Figure 3: Growth of C. sakazakii and Bifidobacteria species incubated
at room temperature.
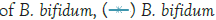
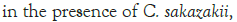
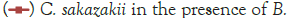
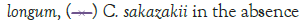
In Figure 1, it can be seen that the pathogen underwent very little growth at 4°C over 8 hours. Their numbers did however increase by an average of 0.3 log cycles. The probiotic cultures showed absolutely no growth in this period. The fact that there was little if any production of secondary metabolites and organic acids that have been shown to inhibit the growth of pathogens such as E. coli, Shigella and Salmonella typhi (Figure 1) [9].
The fact that the growth of C. sakazakii occurred at 4°C is contrary to the findings of Breeuwer et al., Lihono et al. and Osaili et al., which state that there should be no growth at this temperature. Growth should only occur at temperatures of 5.5°C and upwards [17,18].
Growth can also be attributed to the fact that the inoculated RIMF was placed in the fridge directly after reconstitution. This happened at between 50°C and 60°C as per the manufacturer’s instructions. This would mean that the bacteria would have started at these non-permissive temperatures [18,19] but quickly cooled into a temperature range where growth could occur, and then later entered a once again non-permissive cooler temperature. This is shown in Figure 1. By an increased growth between 2 hr-6 hr with a decline in growth from 6 hr-8 hr. This means that the RIMF would have only been at a temperature that allowed the growth of the culture for a brief period of about 4 hours, and this is reflected in Figure 1. In Figure 4, which shows the pH levels correlated to Figure 1, it can be seen that there were no statistically relevant changes in pH. There was a very slight decrease in pH with the two samples containing B. bifidum. The low levels of free H+ ions correspond to the fact that little growth was seen in the probiotic cultures, where a concurrent drop in pH is expected to occur as a result of the production of inorganic acids [20].
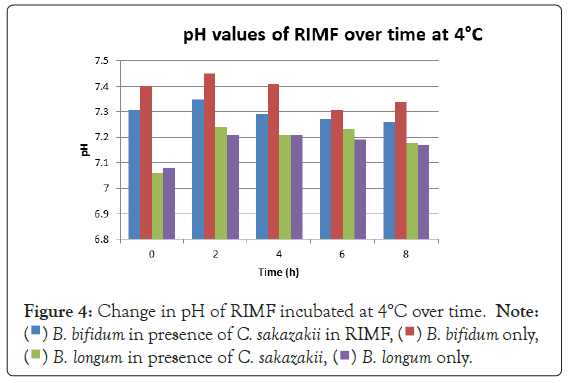
Figure 4: Change in pH of RIMF incubated at 4°C over time.
Figure 2 shows that the growth of the cultures increased when incubated at 37°C as these cultures grow at body temperature [1,18-22]. The pathogen underwent an average increase in numbers by about 1 log cycle while the probiotic increased by slightly more. B. longum outgrew B. bifidum significantly in the presence of C. sakazakii with an increase of 2 log cycles as opposed to less than half a log cycle. The probiotic had however no statistically relevant (<10%) effect on the growth of the pathogen, which can be explained by the temperature alone (Figure 2) [15,17,19].
Figure 5 shows a concurrent drop in pH and growth of the probiotic cultures. The pH dropped considerably more in the samples containing B. longum. This correlates with the higher growth seen in Figure 2 and coincides with the work of Lankaputhra and Shah (1995).
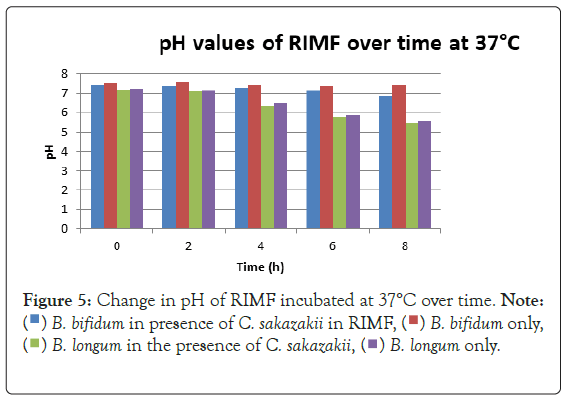
Figure 5: Change in pH of RIMF incubated at 37°C over time.
Figure 3 shows that at room temperature the pathogen underwent an average increase in numbers of 1.5 log cycles in the presence and 1.2 log cycles in the absence of the probiotic. The probiotics achieved an average growth of just above 1 log cycle. The cultures in the sample containing B. longum again achieved significantly more growth (2 log cycles) higher than the sample incubated with B. bifidum for the time measured. The growth of B. longum coincides with the growth of C. sakazakii when incubated together. This did however not occur when C. sakazakii and B. bifidum were incubated together. In Figure 6, it can be seen that the samples showed only a very slight drop in pH except in the sample incubated with C. sakazakii and B. longum. This drop in pH coincides with the growth of B. longum and is due to the production of organic acids as secondary metabolites [20]. The growth rate was lower than at 37°C which coincides with the results of Breeuwer et al., Osaili et al. and Lihono et al. (Figure 3).
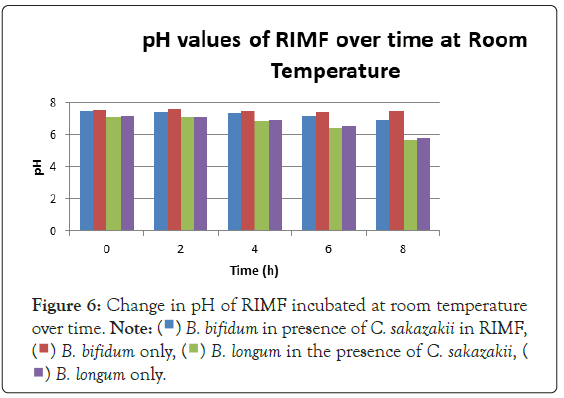
Figure 6: Change in pH of RIMF incubated at room temperature
over time.
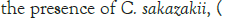

The general objective of this study was to evaluate the effect that the probiotic B. bifidum LMG 11041 had on the growth and survival of C. sakazakii in RIMF. The goal was to examine what effects the presence of the probiotic will produce on levels of the pathogen when they were incubated together for a set period in infant milk formula.
B. bifidum has been shown to inhibit the growth of pathogens such as E. coli, Shigella and Salmonella typhi through the production of organic acids, which results in a high concentration of hydrogen ions in the growth medium [9]. The largest difference in viable numbers was found at 4°C where the presence of the probiotic allowed the pathogen to multiply to significantly higher (above 10%) levels than in the absence of the probiotics. The probiotics and pathogen had similar overall increases at 4°C when incubated together while the pathogen alone had an increase in levels of approximately half their numbers. This shows that there must be some kind of protective effect inferred on the pathogen by the presence of the probiotic at this temperature. This could be due to the production of certain metabolites that stimulate the growth of the pathogen [3] or certain layers that protect or cause the organisms to aggregate [21] offering some protective measure.
The fact that the growth of C. sakazakii occurred at 4°C is contrary to the findings of Breeuwer et al., Lihono et al. and Osaili et al. which state that there should be no growth at this temperature. Growth should only occur at temperatures of 5.5°C and upwards (Figure 4) [17,18].
Growth can also be attributed to the fact that the inoculated RIMF was placed in the fridge directly after reconstitution. This happened at between 50°C and 60°C as per the manufacturer’s instructions.
This would mean that the bacteria would have started at these non-permissive temperatures [18,19] but quickly cooled into a temperature range where growth could occur, and then later entered a once again non-permissive cooler temperature. This is shown in Figure 1 that by growth between 2 hr-6 hr with a decline in growth from 6 hr-8 hr. This means that the RIMF would have only been at a temperature that allowed the growth of the cultures for a brief period of about 4 hours, and this is reflected in Figure 1.
The highest growth was seen at 37°C in Figure 2 which corresponded to findings by researchers elsewhere [15,17,19]. The growth of C. sakazakii in the presence and absence of B. bifidum was within the 5% threshold of each other. This is the parameter that this experiment regards as being the same. This shows that the presence of the probiotic did not affect the growth and survival of C. sakazakii in the RIMF at this temperature (Figure 5).
The higher growth rate at this temperature is attributable to the fact that the organisms both reside inside the human body [18,22]. B. bifidum also proliferated at this temperature but the increase was not as marked as that of C. sakazakii.
A lower growth rate was observed at RT which also coincides with the results of Breeuwer et al., Osaili et al. and Lihono et al. The growth of the cultures showed the same trend at this temperature as shown in Figure 3. The growth of C. sakazakii in the presence and absence of B. bifidum was statistically the same showing at 37°C that the presence of B. bifidum did not affect the growth of C. sakazakii. The growth of B. bifidum at this temperature was however higher than that of C. sakazakii. The C. sakazakii cultures showed a steady exponential growth curve. This can once again be attributed to the temperature of reconstitution which would have initially been non-permissive for growth (Figure 6).
In the case of B. bifidum, the extended phase with no growth could have also been exacerbated by the fastidious and anaerobic nature of this organism. Their anaerobic nature makes their handling in the laboratory set-ups to be troublesome [23]. It is clearly shown that the rate of change in pH is proportional to the number of viable organisms when comparing Figures 3 and 4.
The drop in pH that coincided with a rise in numbers of B. bifidum at 37°C and the higher drop in pH that occurred whenever the probiotic was present is attributable to the fact that the organism produces lactic in addition to other organic acids from utilizable carbon sources [9,24]. No statistical linkage between the correlated averages up to the 5% threshold that is considered the minimum for inferring accurate conclusions from the data in this experiment was found at RT or 37°C.
A significant interaction was found above the 10% mean threshold level at 4°C. Further differences between the numbers of organisms in the experiment and control were statistically negligible, showing there was no significant interaction between the experiment and control data sets. Only statistically relevant counts (30> × >300 were used), this was almost exclusively at the 10-3 dilution. An average of the experiments were used and the 9 different data points were not considered separate for statistical purposes.
The infective dose of C. sakazakii is very low, such that levels as low as 3 CFU/100 ml are sufficient to cause an infection [25-30]. Since the results show growth after reconstitution, this suggests that RIMF should be administered immediately after reconstitution. The results of this study indicate that even storage at refrigerated temperatures in the presence of B. bifidum LMG 11041 is not sufficient to inhibit the growth of this pathogen.
The presence or absence of B. bifidum in RIMF does not significantly influence the growth and survival of C. sakazakii at the 10-threshold level. This difference falls inside the parameters which regard the difference as irrelevant. There is therefore no effective difference in growth and survival of C. sakazakii in either the presence or absence of Bifidobacteria species. The addition of B. bifidum LMG 11041 and B. longum LMG 13197 to RIMF containing C. sakazakii does not change the growth characteristics of the pathogen.
Dr M.S Thantsha (Supervisor) for revision and support. Mr J.P Uys for assistance with the statistical analysis. Mr Iyanda Raselabe for help in the preparation of media and lab duties.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Labuschagne MC, Raselabe CI (2022) Growth and Survival of Cronobacter sakazakii in Reconstituted Commercial Infant Milk Formula in the Presence of Bifidobacterium Species. J Prob Health. 10:283.
Received: 30-Jun-2022, Manuscript No. JPH-22-18777; Editor assigned: 04-Jul-2022, Pre QC No. JPH-22-18777 (PQ); Reviewed: 18-Jul-2022, QC No. JPH-22-18777; Revised: 25-Jul-2022, Manuscript No. JPH-22-18777 (R); Published: 02-Aug-2022 , DOI: 10.35248/2329-8901.22.10.283
Copyright: © 2022 Labuschagne MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.