

Review Article - (2022)Volume 6, Issue 3
The principal components of gut health includes diet, effective structure and function of the GIT barrier (gut mucosal), with effective digestion and absorption of feed and effective immune status of the host and normal and stable micro biota including bacteria, protozoa and fungal zoospores coexist to enable efficient fermentation, absorption and boosting immunity for better host performance demanding holistic considerations. Ruminant’s digestive fermentation results in fermentation end products of Volatile Fatty Acids (VFA), microbial protein and methane production in the rumen. The gut microbiota and their metabolic products also improve nutrient digestion, absorption, metabolism, and overall health and growth performance. Microbial feed supplements as natural growth promoters might play an important role for enhancement of health and productive performance serving in prevention of disease, enhancement of desirable microbial growth in the host environment, stabilization of gut and ruminal pH, altered ruminal fermentation patterns, increased nutrient digestibility and flow of nutrients to the small intestine, improved nutrient retention and reduced stress through enhanced immune response. The gut micro biome of livestock plays a crucial role in feed conversion. The fermentation products are influenced by the type of microbes present, the type of feed, and factors such as genetics and the age of the animal. Feed contributes most to livestock production costs. Improving feed efficiency is crucial to increase profitability and sustainability for animal production. Understanding these functions and interactions will help to develop new dietary and managerial strategies that will ultimately lead to enhanced feed utilization and improved growth performance of animals. Feed efficiency and high performance animals are the crucial goals in livestock farming to cope up the increasing demand driven live stock products.
Gastrointestinal functionality; Microbiota; Immune system; Health; Nutrition
Livestock are vital in feeding the emerging population. Resource use is continually being limited due to this growth, therefore, production of high quality animal protein sources, such as meat, milk and egg, are challenged. Meeting the demands for these consumables, while utilizing fewer resources, is a task the livestock industry is currently facing. The gut and/or rumen microbiome is extensive and serves to provide several metabolic requirements for the animal growth. Recently, a significant amount of research is being driven towards understanding the gut microbiome due to its large effect on metabolic requirements. Feed efficiency and high performance are the crucial goals in host production. Also, the quality of diet along with environmental conditions and health of host need to be considered to achieve these goals [1-3].
Gut microbiota and their metabolic products improve nutrient digestion, absorption, metabolism, and overall health and growth performance of poultry. Ruminants ׳ digestive fermentation results in fermentation end products of Volatile Fatty Acids (VFA), microbial protein and methane production in the rumen. GIT microbiota including bacteria, protozoa and fungal zoospores are closely associated with the rumen fermentation efficiency, and strengthen immunity. Animals are usually regarded as independent entities within their respective environments. However, within an organism, eukaryotes and prokaryotes interact dynamically to form the so-called metaorganism or holobiont, where each partner fulfills its versatile and crucial role. Dense and complex microbial communities reside in the gastro-intestinal tract of host animals, which can be composed of bacteria, protozoa, fungi, archaea, and viruses. Amongst the valuable effects, gastro-intestinal tract microbial communities are aimed to digestion and fermentation of plant polymers, which is of specific significance in herbivorous animals. Moreover, the indigenous gut microbiota is accountable for the synthesis of vitamins, bioconversion of toxic compounds to non-toxic residues, stimulation of the immune system, maintenance of gut peristalsis and intestinal mucosal integrity and plays a barrier role against colonization by pathogens.
Historically, the Gastrointestinal Tract (GIT) was considered an organ solely equipped for the digestion and absorption of nutrients. However, the GIT harbors the largest population of immune cells and microbes that outnumber the entire host cells. Therefore, there is a general consensus that a healthy gut leads to healthy host with optimal performance. The rumen is perhaps the most diverse and complex microbial ecosystem harbored in the GIT of animals. This microbial community provides an evolutionary advantage for ruminants, which allows them to utilize lignocellulosic materials and non-protein nitrogen to produce high quality foods. As a result, ruminants are capable of digesting a wide range of forages, decreasing the competition for human-edible foods. However, rumen microbial fermentation has some drawbacks: Proteolysis carried out by protozoa and certain bacterial species can lead to low nitrogen efficiency and the excess ruminal ammonia, not captured by the ruminal microbiota for their own protein synthesis, is absorbed and excreted to the environment. Similarly, CH4 formation in the rumen by the methanogenic archaea is wasteful in terms of feed energy loss as well as contributes to climate change. Moreover, rumen fatty acid bio hydrogenation by rumen microbes leads to more saturated fat in ruminants' milk and meat in comparison to monogastric animals. Thus, understanding the interactions between the gut microbiome, diet, host genetics and health are key to develop new strategies to meet consumers' demands for better food quality, animal health, and a more environmentally friendly and efficient animal production. The paper aimed to review mongastric and poligastric gut microbial ecology role in health and performance and feeding strategies to enhance the economic returns of livestock farming [4,5].
Objectives
• To review features of microbiome-immunity crosstalk and their roles in health and disease
• To determine the role of GIT microbiota in health and performance in livestock production and assess factors that affect microbial function and possible feeding strategies
Definition of gut microbiota
According to Yeoman and White, microbiome represent the collection of autochthonous microbes occupying a defined environment (e.g., the gastrointestinal tract) while microbiota represent the complete microbial community (autochthonous and allochthonous) that occupies a defined environment (e.g., the gastrointestinal tract). The oral cavity and the gastrointestinal tract of vertebrates are colonized by large numbers of microorganisms, including bacteria, fungi, archaea and protozoa, commonly referred to as the microbiota. Many of the resident bacteria are adapted to the intestinal environment and develop complex interactions with other bacteria and host niches to acquire nutrients. Although the GIT is frequently described simply as ‘‘the gut,’’ it is actually made up of (1) An epithelium; (2) A diverse and robust immune arm, which contains most of the immune cells in the body; and (3) The commensal bacteria, which contain more cells than are present in the entire host organism. Understanding of the crosstalk between ALL of these interrelated components of the gut is what cumulatively makes the gut the basis for the health of animals and the motor that drives their performance. According to Hippocrates 460-370 BC, "all disease begins in the gut and health is determined by the microbiota in the gut!" and also remarked as 'Let food be thy medicine and medicine be thy food’.
The gut microbiota, the largest symbiotic ecosystem with the host, has been shown to play important roles in maintaining intestinal homeostasis. Gut microbiota of animals extensively interact with the host, diet, and gut mucusal and immunity system. The mammalian intestinal tract is the largest immune organ in the body and comprises cells from nonhemopoietic (epithelia, paneth cells, goblet cells) and hemopoietic (macrophages, dendritic cells, Tcells) origin, and is also a dwelling for trillions of microbes collectively known as the microbiota. The homeostasis of this large microbial biomass is prerequisite to maintain host health by maximizing beneficial symbiotic relationships and minimizing the risks of living in such close proximity. Both microbiota and host immune system communicates with each other to mutually maintain homeostasis in what could be called a “love–hate relationship.” Further, the host innate and adaptive immune arms of the immune system cooperate and compensate each other to maintain the equilibrium of a highly complex gut ecosystem in a stable and stringent fashion. Any imbalance due to innate or adaptive immune deficiency or aberrant immune response may lead to dysbiosis and low-grade to robust gut inflammation, finally resulting in metabolic diseases [6-10].
Composition of gut microbiota: The gut microbiota is composed of approximately 1010‐1014 cells, including fungi, bacteria, archaea, protozoa, viruses, and bacteriophages; their genes and their various metabolites were found throughout the gastrointestinal tract. It has co‐evolved with each species to assist with day to day bodily functions, such as digestion, metabolism of xenobiotics, development of mucosal immunity and immunomodulation, and protection against invading pathogens. Results from recent studies characterizing the gut microbiota of various species have demonstrated the range of influences that may affect gut microbiota diversity, including animal strain, obesity, types of enrichment used bedding and housing methods, treatment with antimicrobials, vendor source, specific animal housing, diet, and inter-current disease.
The intestinal tract of mammals is colonized by a large number of microorganisms including trillions of bacteria that are referred to collectively as the gut microbiota. These indigenous microorganisms have co-evolved with the host in a symbiotic relationship. In addition to metabolic benefits, symbiotic bacteria provide the host with several functions that promote immune homeostasis, immune responses and protection against pathogen colonization. The ability of symbiotic bacteria to inhibit pathogen colonization is mediated via several mechanisms including direct killing, competition for limited nutrients and enhancement of immune responses. Pathogens have evolved strategies to promote their replication in the presence of the gut microbiota. Perturbation of the gut microbiota structure by environmental and genetic factors increases the risk of pathogen infection, promotes the overgrowth of harmful pathobionts, and the development of inflammatory disease. Understanding the interaction of the microbiota with pathogens and the immune system will provide critical insight into the pathogenesis of disease and the development of strategies to prevent and treat inflammatory disease.
The composition of the microbiota is largely defined by nutrient requirements of individual bacteria and highly variable at different locations of the intestinal tract. Gut microbiota interacts within themselves, with their host, and with the diet of the host, whereas commensal bacteria play a pivotal role in host health and metabolism, and pathogenic bacteria cause direct or indirect harmful effects. Thus, feed ingredients should be selected to favor gut condition and maintain a balance between the environment, host, and microbiota.
The microbes inhabiting the rumen convert low quality, fibrous, plant material into useable energy for the host ruminant.
Consisting of bacteria, protozoa, fungi, archaea, and viruses, the rumen microbiome composes a sophisticated network of symbiosis essential to maintenance, immune function, and overall production efficiency of the host ruminant. The interplay between the rumen microbiome and the host contributes to variation in many phenotypic traits expressed by the host animal. A better understanding of how the rumen microbiome influences host health and performance may lead to novel strategies and treatments for trait improvement. Furthermore, elucidation of, genetic, and environmental factors that influence rumen microbiome establishment and development may provide novel insights into possible mechanisms for manipulating the rumen microbial composition to enhance long-term host health and performance. The potential for these tiny but mighty rumen microbes to play a role in improving livestock production is appreciated despite being relatively obscure.
The microbial species recently used in probiotics mixtures are many and varied, grouped. As Bacteroides, Clostridium, Bifidobacterium, Eubacterium, Lactobacillus, Enterobacteriaceae, Streptococcus, Fusobacterium, Peptos treptococcus and Propionibacterium are used in monogastric animals.
In polygastric animals, the rumen is the most vital microbial ecosystem with the majority of fiber degrading groups belonging to Fibrobacter, Ruminococcus, Butyrivibrio and Bacteroides together with major groups such as Prevotella, Selenomonas, Streptococcus, Lactobacillus and Megasphaera.
Moreover, the rumen maintains some anaerobic fungi, ciliate protozoa and a large number of methanogens to keep normal rumen microenvironment as indicated in Table 1. Taxonomic assignments are performed using a variety of tools that assign each sequence to a microbial taxon (bacteria, archaea, or lower eukaryotes) at different taxonomic levels from phylum to species.
| Microorganisms | Animals | Common Benefits |
|---|---|---|
| Pig | ||
| E. faecalis | Improve colostrum quality, milk quality and quantity | |
| E. faecium | Increase litter size and vitality | |
| Bacillus cereus | Increase piglet weight | |
| B. subtilis | Reduce risk of diarrhea | |
| B. licheniformis | Improve feed efficiency, diet digestibility and meat quality | |
| L. johnsonii | Limit constipation | |
| L. reuteri | Decrease stress | |
| L. acidophilus | ||
| S. cerevisiae | ||
| Poultry | ||
| L. animalis | Increase body weight gain | |
| L. fermentum | Reduce mortality | |
| L. salivarius | Increase carcass quality decreasing contamination | |
| L. acidophilus | Increase bone quality | |
| S. faecium | ||
| L. reuteri | ||
| E. faecium | ||
| S. cerevisiae | ||
| Bacillussp | ||
| Veal Calf | ||
| S. cerevisiae | Promote weight gain and optimal maturation of rumen microbiota limiting acidosis | |
| L. acidophilus | Increase feed efficiency, milk yield, quality and digestive safety at weaning | |
| B. pseudolongum | Reduce risk of pathogen colonization and limit shedding of human pathogens | |
| L. animalis | ||
| L. paracasei | ||
| Horse | ||
| Lactobacillus pentosus | Improve diet digestibility, milk quality and quantity | |
| L. rhamnosus | Limit diarrhea | |
| L. acidophilus | Avoid hindgut disorders (acidosis, colic) | |
| L. plantarum | Limit stress | |
| L. casei | (Transportation, race etc.) | |
| S. boulardii | ||
| S. cerevisiae |
Source: ASML, et al.
Table 1: Some microbial species of potential use as livestock probiotics with their benefits.
Techniques of gut microbial determination: Identifying certain metabolic pathways and further research into these pathways may determine the best diet for bovine ruminants in order to minimize energy loss, reduce methane production and increase nitrogen utilization efficiency. Examining the rumen micro biome can identify the effects of diet on the microbiome and in turn, the effects on milk yield, protein percentages, urea percentage (used as a NPN indicator) and milk protein yield. The composition of proteins found in milk can have a profound effect on milk process ability, thus identifying pathways which may influence milk composition and improve process ability is important.
There are different techniques used to identify and characterize intestinal microbiota such as traditional culture based and modern molecular based culture independent high throughput sequencing.
Culture dependent approaches: Culture dependent techniques rely on various selective and enrichment culture conditions in order to replicate the microbes’ natural environment. Culturing anaerobes is quite difficult due to the need to exclude oxygen, the slow growth of the microbes and the complexity of other growth requirements used a continuous culture system in order to replicate the rumen environment. This technique, along with similar techniques, was used for the enumeration and identification of rumen microbes. Traditional methods of classifying rumen bacteria were based on the standard bacterial identification methods; morphology, shape and gram stain. Nutritional requirements and fermentation end products were also used as a means of classification. Roll tubes came to be employed to grow and isolate anaerobic species, and were used instead of conventional agar plates [10-20].
Culture independent approaches: Culture independent methods, or, more specifically DNA based methods of identification and detection of microorganisms, allow the examination of microbial communities at a molecular level. Conventional (classical culture based techniques, such as isolation, enumeration and nutritional characterization) and modern molecular techniques (the use of small sub-unit rDNA analysis e.g. 16S rDNA (phylogenetic composition) using RTPCR, oligonucleotide probes, denaturing Gradient Gel Electrophoresis, Northern Blot Analysis, etc.) that are currently in use for studying the gut microbial ecology of ruminants, metagenomics (functional capability), meta-transcriptomics (functional intent) and metabolomics (metabolic impact) are providing a more comprehensive understanding of the changes in the composition of the intestinal microbiota and their secreted metabolites, providing valuable information on the GIT. Culture independent approaches rely on bioinformatics tools to handle large datasets. Among the molecular techniques, the Terminal Restriction Fragment Length Polymorphism (TRFLP) was used to compare and contrast microbiota in the duodenum, jejunum, ileum, and ceca. The advancement of DNA/RNA, proteins, and metabolite analytical platforms, combined with increased computing technologies, has transformed the field of microbial community analysis. The microbial census is established using molecular methods relying predomina analysis of 16S rRNA genes, 18S rRNA marker genes and genomic regions, amplified and sequenced from given biological samples.” The “S” in 16S is a non-SI unit for sedimentation rate and stands for the Svedberg unit. The Svedberg unit offers a measure of particle size based on its rate of travel in a tube subjected to high g force.
Metagenomic analysis allows the description of a microbial community by high throughput sequencing technology. Methods associated with this type of analysis include 16S rRNA and Internal Transcribed Spacer (ITS) amplicon sequencing, used for bacterial and fungal communities, respectively, or, shotgun sequencing, where DNA fragments are sequenced randomly, regardless of the microbe from which they came. Targeting of the mcrA gene has also been suggested in recent studies as a means of identifying methanogens. Strategies used by each platform determine quality, quantity and bias of sequenced data. Individual species can be quantified by using Fluorescent In Situ Hybridization (FISH), dot/slot blot hybridization, or Q-PCR. Techniques such as metagenomic shotgun sequencing provide a more in-depth understanding of microbiota functionality in specific environments with strong differentiation between treatments microbiota profile. Similarly, next generation sequencing has made it possible to determine microbiota dynamics with increased coverage and accuracy. Sequencing also showed that Prevotella, Butyrivibrio and Ruminococcus were the most dominant bacteria in the rumen, and that community structure is affected by changes in the diet of the host. Additionally, 16S rRNA sequencing only gives information on bacterial populations, not fungal, viral, protozoa or amoeba, all of which are believed to play an important role in the rumen. The abundance and function of microbiota at different sites of the Gastrointestinal Tract (GIT) (foregut: stomach, duodenum, jejunum and ileum; hindgut: Cecum, ventral colon, dorsal colon, and rectum) of healthy adult donkeys was investigated mainly based on 16S rRNA gene sequencing and Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis.
Role of gut microbiota
Commensal gut microbiota plays a decisive role in maintaining the normal physiology of host animals. Some of the major roles are to help direct the normal formation or development of gut structure and morphology, boost immune responses, offers protection from luminal pathogens, as well as play an active role in digestion and utilization of nutrients. The microbiota positively and negatively impacts host physiology and performance in many important. Gut microbiota also has some direct and indirect harmful effects on chickens such as decrease digestibility of fat, increase cell turnover rate, production of toxic metabolites from the protein fermentation and may also lead to poor growth performance. The microbiome in the rumen is the first to come into contact with the biomass of the forage and its main purpose is to decompose into smaller particles or compounds. With the gradual increase in knowledge about the microbiome, there is a chance to manipulate it so that the animal continues to live in a symbiotic relationship with it, while reducing greenhouse gas emissions to the environment as well as increasing feed efficiency.
Beneficial roles of gut microbiota: Gut microbiota provides nutritional compounds to the host in the form of fermentation end products and other secreted products such as SCFAs, specialized enzymes, amino acids, B and K vitamins and absorption of ions. Commensal bacteria generate SCFAs such as acetate, propionate, butyrate, and lactate in the GIT of chickens. These SCFAs have their specific role in the GIT such as contribution to energy by gluconeogenesis and reducing undesirable bacterial species in the cecum. SCFAs also stimulate gut epithelial cell proliferation, differentiation and increase the villus height, thereby increasing the absorptive surface area. Acetate and propionate also act as an energy substrate for tissues. Recently, xylanase genes are isolated and overexpressed from the cecum of chickens which can degrade and digest complex substrate like non-starch polysaccharides which will encourage nutritionist and researchers to explore alternative feedstuffs to incorporate in large scale industrial production.
Feed digestion and physiology: Many animals across a wide range of orders have a portion of their digestive tract adapted to accommodate a microbial population which aids in digestion and provides a variety of nutritional and health benefits. Microbial populations have been described in the gut of herbivores, omnivores and carnivores and in all zoological classes. This complex, mixed, microbial culture (comprising bacteria, ciliate and flagellate protozoa, anaerobic phycomycete fungi and bacteriophage) forms a closely integrated ecological unit with each other and the host animal, as well as playing a vital role in the nutritional, physiological, immunological and protective functions of the host. Development of microbial populations in the digestive tract of higher animals commences soon after birth and involves a complex process of microbial succession and many microbial host interactions which, eventually resulting in dense, stable microbial populations inhabiting characteristic regions of the gut.
The ruminant foregut or stomach has evolved into three pregastric fermentation chambers (rumen, reticulum and omasum) of which the rumen is by far the largest. Ingested plant material is hydrolysed and fermented in the rumen, and microbial cells and undigested plant particles pass into the abomasum where gastric digestion begins (Figure 1). The most distinctive feature of ruminants, rumination, where foregut digesta is regurgitated, richweed and swallowed in a frequent regular pattern repeated up to 500 times per day and enables reduction in particle size (comminution) and exposure of maximal surface area to microbial attack. The mutualistic microbial fermentation is based on digestion of the Fermentative microbes, mainly bacteria; hydrolyse plant polymers (starch, cellulose, hemicellulose, pectins and protein) to short oligomers and monomers. These soluble substrates are transported into the microorganism by specific transport mechanisms and fermented, resulting in synthesis of microbial cells and production of fermentation end products (acetate, propionate, butyrate, carbon dioxide and hydrogen, methane, ammonia and occasionally lactic acid). Hydrogen and formate are produced by many microbes in the rumen where the hydrogen is quantitatively converted to methane by methanogenic archaea, resulting in undetectable or low levels of free hydrogen in the gas phase. Although acetogenic and syntrophic bacteria that also consume hydrogen have been isolated, they are of minor quantitative importance in the rumen. Some of the change in free energy is used to drive microbial growth, but heat is also evolved.
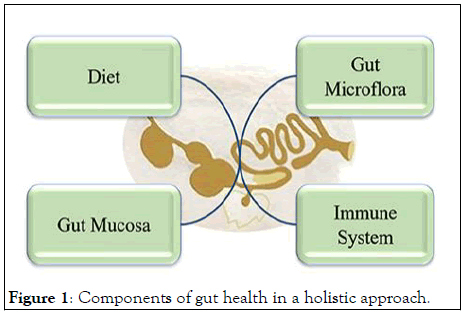
Figure 1: Components of gut health in a holistic approach.
The molar proportions in which the principal volatile fatty acids are formed in the rumen are 65 acetic, 20 propionic, 12 butyric and 3 higher and branched chain volatile fatty acids. The volatile fatty acids provide 60–80 percent of the daily metabolizable energy intake in ruminant animals and provide the energetic foundation for the mutually beneficial association between the rumen microbes and the host animal. It follows therefore that ruminants are characterized by low blood glucose levels and rely heavily on gluconeogenesis for provision of glucogenic precursors. The quality and quantity of rumen fermentation products is dependent on the digestive interactions between amount and quality of feed ingested and the types and activities of the rumen microbes, which in turn, has an enormous impact on nutrient output and performance of ruminant animals.
Protozoa engulf bacteria, fungi and other smaller protozoa. This activity plays a significant role in intraluminal nitrogen recycling and the efficiency of protein synthesis in the rumen. Protozoa play a major role in the ingestion of particulate protein, including plant (supplementary) protein and a lesser role in uptake of soluble protein, peptides and amino acids. Protozoa have mixed protease activity similar to the bacteria and rapidly deaminate amino acids. Isolation and characterization of ammonia hyper producing bacteria and investigation of their role in rumen fermentation of peptides and amino acids are current research topics. Fungi also have proteolytic activity, mostly trypsin like metalloprotease. Recent evidence suggests a role for plant proteases in initial proteolysis of plant proteins.
Microbial gut role in feed intake and feed conversion efficiency: Feed efficiency is an important factor in both the profitability and sustainability of livestock production facilities, strategies for trait improvement are in demand. When feed conversion is high, animals require less feed to put on weight, or produce eggs or milk. Feed costs are a large part of costs for farmers, so improving feed conversion directly improves financials for farmers. Better feed conversion also means that animals produce less waste and the environmental “footprint” is reduced. The gut microbiome of livestock plays a crucial role in feed conversion. Gut microbial fermentation converts feed to nutrients that are used by the animal. The fermentation products are influenced by the type of microbes present, the type of feed, and factors such as genetics and the age of the animal. There are also indirect effects related to improved immunity: healthy animals put on weight efficiently. Feed contributes most to livestock production costs. Improving feed efficiency is crucial to increase profitability and sustainability for animal production. Host genetics and the gut microbiota can both influence the host phenotype. Aspects of the rumen microbiome are associated with host phenotypic variation in feed efficiency, indicating there may be potential for rumen microbes to serve as indicators for feed efficiency. Selection for better feed efficiency may also result in lower methane production. Reported high enrichment of the methanogenesis pathway in lowly feed efficient cattle (high RFI) and hence greater methane production.
The microbes inhabiting the rumen convert low-quality, fibrous, plant material into useable energy for the host ruminant. Consisting of bacteria, protozoa, fungi, archaea, and viruses, the rumen microbiome composes a sophisticated network of symbiosis essential to maintenance, immune function, and overall production efficiency of the host ruminant. Microbial genes related to cellulose and hemicellulose degradation, vitamin B12 synthesis, and amino acids metabolism were associated to enhanced feed conversion efficiency (FCR or RFI), while those involved in nucleotide sugars metabolism, pathogen LPS synthesis, cAMP resistance, and degradation of toxic compounds were associated with inefficient feed conversion. Thus, our results provide a deeper understanding of the potential influence of the rumen microbiome on the feed conversion efficiency of its host, highlighting specific enzymes involved in metabolic pathways that reflect the complex functional networks impacting the conversion of feed into animal products such as meat.
Feed efficiency is a crucial parameter in host production, given both its economic and environmental impact. The gut microbiota plays an essential role in nutrient digestibility and is, therefore, likely to affect feed efficiency that microbial community contribute to shaping host productive parameters. The link between the porcine intestinal microbiota and growth and feed efficiency, and suggests microbiota targeted strategies to improve productivity. Possible microbial signaling routes linked to superior growth and FE includes increased intestinal propionate production and reduced inflammatory response. In summary, the bacterial taxa and/or metabolic pathways identified here could be used as biomarkers for FE/growth in pigs, the taxa exploited as probiotics or the taxa/functionality manipulated via dietary/breeding strategies in order to improve productivity in pigs.
In response to a rapidly growing human population and the economic burden of feed costs, a major goal of the livestock industry is to improve feed efficiency. Feed efficiency describes the efficacy at which the conversion of feed to useable product occurs. Because feed efficiency is influenced by many factors (that also influence the rumen microbiome (e.g., diet, stage of development, energy availability), it is likely that there is also a link between feed efficiency and the rumen microbiome. Furthermore, the conversion of feedstuffs to usable energy depends on assimilation of nutrients, which is contingent upon fermentation by the rumen microbes. Both microbial species diversity and richness have been associated with divergence in feed efficiency, based on RFI estimates, in cattle and sheep. Because feed efficiency is affected by diet, many of the rumen microbial differences associated with feed efficiency may be driven, in part, by diet. In addition to rumen microbiome population dynamics associated with feed efficiency, the functional aspects of these populations may also help describe the variation in energy harvesting. Significant differences in rumen metabolic activity associated with divergence in feed efficiency have been reported where concentrations of propionate, butyrate, valerate, and isovalerate were higher in more efficient animals as was total concentration of the short chain fatty acids.
Metatranscriptomic analysis of the rumen microbial community has shown significant differences in both bacterial and archaeal community structure between high residual feed intake groups and low residual feed intake groups. The higher abundance of family Lachnospiraceae in high RFI groups may indicate an increase in butyrate metabolism, therefore, a resulting impact on feed efficiency. In contrast, the order Methanomassiliicoccales were more abundant in low RFI animals. Members of this order can use methylamine as an energy source, which may be harmful to the host, to produce additional ammonium during methanogenesis.
Methane is generated in the foregut of all ruminant animals by the microorganisms present. Dietary manipulation is regarded as the most effective and most convenient way to reduce methane emissions (and in turn energy loss in the animal) and increase nitrogen utilization efficiency.
There have been many attempts to minimize energy loss as a result of methane production. A novel, promising approach, is the use of probiotics. Probiotics present health benefits for the host when provided in sufficient amounts. Explored the use of Bacillus licheniformis as a novel probiotic to reduce enteric methane emissions. Probiotics are defined as live microorganisms that can confer a health benefit for the host when administered in appropriate and regular quantities. The isolation and identification of microorganisms is the first step in the selection of potential probiotics from gut, feces and milk of respective animals. The mechanisms of action of probiotics include the inhibition of pathogen growth by competition for nutritional sources and adhesion sites, secretion of antimicrobial substances and toxin inactivation. Consequently, the primary interest in the application of probiotics has been in the prevention and treatment of gastrointestinal infections and antibiotic associated animals’ diarrheal diseases. Two of the most important objectives for using probiotics in animal feed are to maintain and improve the performance of the animal, and prevent and control enteric pathogens.
Gut microbiota and health: The GIT micro biome shields the host animal against environmental threats and diseases through various mechanisms including the modulation of the immune system and promoting health and productivity in ruminants. The perturbation of this commensal microbiota can result in GIT disorders such as rumen acidosis, bloat, nutrient toxicity, and diarrhea. These health problems, which represent major welfare and economic concerns in the current intensive production systems, have been conventionally addressed through the “one-pathogen one-disease” approach. However, there is increasing evidence showing the importance of symbiotic micro biomes as major players modulating and minimizing the incidence of these GIT disorders as well as mastitis and respiratory disease. The interaction between the microbes and their host gives new insights into the ways the GIT microbiota can affect host stress, metabolic efficiency, or resistance to disease.
The Gastrointestinal Tract (GIT) microbiomes of production animals are now firmly established as a key feature underscoring animal health, development, and productivity. In particular, early gut colonization is critically important to the morphological and immunological development of the GIT, development of a functional fermentative environment, and neonatal resistance to pathogenic challenge. Although perturbations of an animal’s GIT microbiome at any age can have profound consequences, perturbations during early GIT development can be particularly severe and result in significant and long-lasting sequelae. In ruminants, early rumen development is vital for efficient fermentation that converts plant materials to human edible food such as milk and meat. Early supplementary feeding of lambs before weaning is important to meet their nutritional needs, promote the development of rumen and improve performance. In natural settings offspring of monogastrics derive their gut associated microbiota through vertical transmission during the birthing or hatching process. Although hens externalize eggs through their vent, a common external opening for excretion of fecal matter, the practice of cleaning eggs pre-hatch removes many co-evolved avian microbes leaving newly hatched chicks to colonize with environmentally derived non-host adapted microbiota.
Microbial colonization is critical to the development of GIT morphology, maturation of the immune system, and development of the fermentative environment. For ruminants, ruminal pH gradually decreases over the first eight weeks, commensurate with increasing SCFA concentrations, before stabilizing at approximately ten weeks of age. This corresponds to an increased buffering capacity of the rumen. The relative concentrations of the various SCFAs are affected by microbiome composition. The microbially produced SCFA, butyrate, positively increases the length and width of GIT papillae along with crypt depth. Microbial colonization of the gut also alters the types of mucins produced by GIT goblet cells and plays a critical role in developing and modulating the immune system. The colonizing microbiota’s effect on systemic immune function has important implications in the host’s ability to respond to pathogenic challenge.
Normal microbiota stimulates development of intestinal host defenses, including the mucus layer; the epithelial monolayer; and the lamina propria, a system of immune cells that underlie the epithelium. The mucus layer segregates both normal and pathogenic microbes away from the animal tissues. The epithelium provides a barrier to entry into the animal tissues in cases where the mucus layer has been crossed. The underlying network of immune cells provides antibodies, cytotoxic and helper T cells, and phagocytic cells. These immune cells combat not only pathogenic bacteria and their toxins, but also the overgrowth of or inappropriate attachment by the normal micro biota. The intestinal immune system develops in parallel with the development of the normal microbiota. For example, introduction of even a single species of commensal bacteria into germ free animals can stimulate the development of the secretory IgA system, induce Major Histocompatibility Complex (MHC) class II expression in intestinal epithelial cells, and restore proper intestinal T cell ratios. Furthermore, these changes only occur in the intestinal locations where colonization has occurred. The association between reduced diversity and disease indicates that a species rich gut ecosystem is more robust against environmental influences, as functionally related microbes in an intact ecosystem can compensate for the function of other missing species. Consequently, diversity seems to be a generally good indicator of a “healthy gut.” But recent interventional studies indicate that major increases in dietary fibre can temporarily reduce diversity, as the microbes that digest fibre become specifically enriched, leading to a change in composition and, through competitive interactions, reduced diversity.
In many countries, grazing herbivores are exposed to toxic forages. Animals that are foregut fermenters can often detoxify or reduce the toxicity of these plants by microbial metabolism although microbial biotransformation of certain compounds in the gut can also enhance the toxicity. An important reason for the evolution of foregut fermentation is detoxification of phytotoxins and mycotoxins. Phytotoxins occur in many common feeds, including grains, protein supplements and forages. They range from tannins, alkaloids, goitrogens, gossypol, saponins, glucosinolates, mimosine and cyanogens to nitrate and oxalate. The rumen microbiota provides a protective function and effectively modifies or degrades a wide variety of toxic compounds. However, prior exposure of rumen bacteria to many of the plant toxicants increases the rate of subsequent detoxification and thus adaptation is an important factor to consider. Utilization of the toxin as a source of energy is usually the most important factor driving adaptation in the rumen. However, the toxin degrading population can also be selected for indirectly and enriched by manipulating the diet to provide other energy yielding substrates, preferred sources of nitrogen, growth factors and substrates that can act as electron donors or acceptors in the metabolism of the toxin. This ability can be modified and deliberately managed as a system to detoxify feedstuffs both naturally by adaptation or inoculation, and through modern genetic engineering technology.
There is a delicate balance between the ruminant animal and its microbial flora. Changes in this delicate balance can cause metabolic disorders leading to decreased nutrient availability preventing the animal from reaching its genetic potential, causing huge economic losses in the agricultural industry. Understanding this host-microbe relationship will provide opportunities to make effective management decisions to improve animal health and productivity and to manipulate the microbial ecosystem to increase animal nutrition.
Yeoman and White summarized that beyond nutritional contributions; the GIT Microbiome can attenuate toxins and other xenobiotic, increase the host’s resistance to pathogenic bacteria, and play critical roles in the maturation of the immune and endocrine systems. The collective colonizing microbial communities relate to the host’s nutrition, development, health, and disease. The current major directions of research into the GIT microbiomes of production animals focus on improving animal health and thereby reducing animal morbidity and mortality, reducing the potential for impacts of animal production on human health, reducing the environmental impacts of animal production, defining the microbial determinants of animal productivity, and improving the health attributes and quality of meat and milk products.
Improved ruminant’s health and its productive performance are a primary goal of producers and other value chain actors of animal production sector. Microbial feed supplements as natural growth promoters might play an important role for enhancement of health and productive performance serving in prevention of disease, enhancement of desirable microbial growth in the host environment, stabilization of gut and ruminal pH, altered ruminal fermentation patterns, increased nutrient digestibility and flow of nutrients to the small intestine, improved nutrient retention and reduced stress through enhanced immune response.
Harmful roles of gut microbiota: Dysbiosis of the gut Microbiome is caused by the imbalance between the commensal and pathogenic microbiome. The commensal microbiome regulates the maturation of the mucosal immune system, while the pathogenic microbiome causes immunity dysfunction, resulting in disease development. The gut mucosal immune system, which consists of lymph nodes, lamina propria and epithelial cells, constitutes a protective barrier for the integrity of the intestinal tract. The composition of the gut microbiota is under the surveillance of the normal mucosal immune system. Inflammation, which is caused by abnormal immune responses, influences the balance of the gut microbiome, resulting in intestinal diseases.
Intestinal microbes decrease fat digestibility by DE conjugating bile acids. Bile acids and their salts are required to emulsify and absorb fat in the intestine. Catabolism of the bile salts in the gut by a variety of microbiota causes a decrease in lipid absorption and produces toxic products that inhibit the growth of chicken. Microbiota alters the intestinal morphology, cell turnover rates, and mucus secretion. Conventionally raised animals have higher small intestine weight due to its thicker walls, longer villi, and deeper crypts which allow infiltration of immune and connective tissue as compared to germ free animals. An increase in thickness of the GIT wall and connective tissue decreases the nutrient uptake.
The negative effects include immune costs, competition for nutrients and the production of toxic amino acid catabolizes decreased fat digestibility, and altered intestinal morphology and function. The macrobiotic plays a key role in host immune development. However, there is inherent inefficiency when immune stimulation is maintained at a chronic level, as appears to be the case in conventional vs. germ free animals. It has been reported that the IgA secreted across the intestinal mucosa accounts for >70% of total antibody production in an animal. Putrefaction produces many harmful and toxic compounds, which in high concentrations may have adverse effects on chicken growth and performance. The protein fermentation products include amines, indoles, phenols, cresol and ammonia, which can all negatively affect host or cell health. All actions to reduce the amount of ileal bypass protein potentially also reduce production of toxic protein fermentation metabolites in the caecum. Enzymes which facilitate protein digestion in the upper intestine and soluble carbohydrates resistant to ileal digestion both reduce caecal putrefaction. In the distal intestine,saccharolytic fermentation is preferred and putrefaction accelerates only when utilizable carbohydrates are depleted. Soluble oligo and polysaccharides are produced in situ by nonstarch polysaccharide degrading enzymes and can also be added directly to the diet as health promoting prebiotics.
Factors affecting gut microbiota: The GIT is the largest group of organs in the body. It is not only the site of digestion and absorption of dietary nutrients but provides protection against pathogens and toxins. Moreover, it hosts a large population of microbiota and immune cells. Thus, a healthy intestinal tract is of utmost importance for overall sound health and improved productivity of animals. Gut health is “a steady state where the microbiome and the intestinal tract exist in symbiotic equilibrium and where the welfare and performance of the animal is not constrained by intestinal dysfunction”. This definition combines the principal components of gut health includes diet, effective structure and function of the GIT barrier (gut mucosal) and normal and stable microbiota, with effective digestion and absorption of feed and effective immune status of the host. Conway proposed that gut health is the function of three major components: the diet, the mucosa, and the commensal microbiota. Later, Montagne, et al. elaborated that it includes a diet that would provide sufficient nutrients, mucosa that maintains the gut integrity, and a microbial community that maintains a balanced, healthy environment. Since the GIT of pigs and poultry contains about 70% of total body immune cells, it should be included in the definition of “intestinal health.” Thus, we suggest that intestinal health should be considered in a holistic way including the diet, mucosa, microbiome, and immune system (Figure 1).
The immediate post weaning period is one of the most stressful phases in a pig's life, and during this period, piglets are usually exposed to environmental, social and psychological stressors which have direct and indirect effects on gut health and overall growth performance. Husbandry practices in the swine barn generally include nutrition and management practices, maintenance of hygienic standards and disease prevention protocols, and animal welfare considerations. Poor husbandry practices could result in reduced feed intake, stress and disease conditions, and consequently affects gut health and performance in weaned piglets. Reduced feed intake is a major risk factor for impaired gut structure and function and therefore a key goal is to maximize feed intake in newly weaned piglets as indicated in Figure 2. In weaned piglets, crowding stress could reduce pig performance, favour the proliferation of pathogenic bacteria resulting in diarrhea, stimulate immune response and interfere with beneficial microbial activities in the gut. Sanitation conditions in the swine barn plays an important role for optimal piglet performance, because unclean conditions reduced growth performance, shifted nutrient requirements to support the immune system and negatively affected the gut morphology in weaned piglets. Appropriate biosecurity measure need to be designed to prevent disease entry and spread within a swine operation, which in turn helps to keep a pigs and piglets healthy. Collectively, husbandry practices relating to feeding and nutrition, animal welfare, biosecurity and disease prevention are important determinants of gut health and piglet performance. Thus, it is suggested that adopting high husbandry practice is a critical piece in strategies aimed at raising pigs without the use of in-fee antibiotics.
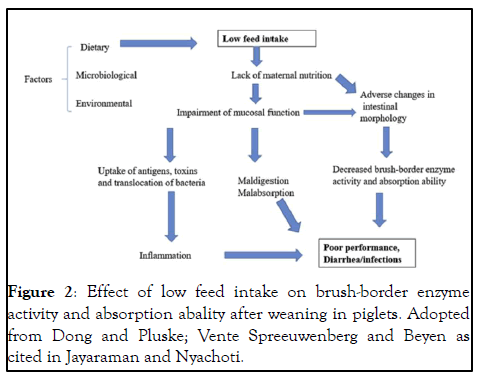
Figure 2: Effect of low feed intake on brush-border enzyme activity and absorption abality after weaning in piglets. Adopted from Dong and Pluske; Vente Spreeuwenberg and Beyen as cited in Jayaraman and Nyachoti.
The GIT consists of hemopoietic cells (macrophages, dendritic cells, and T-cells), non-hemopoietic cells (epithelia, Paneth cells, and goblet cells)). And the microbiome (bacteria, archaea, protists, fungi, and viruses) all of which contribute to gut health. The innate and adaptive immune systems constantly communicate with the microbiome to maintain homeostasis. Any imbalance in the immune system or the microbiome can lead to dysbiosis, resulting in increased susceptibility to various diseases.
The intestinal mucosa is composed of the digestive epithelium with its specific structure, the Gut-Associated Lymphoid Tissue (GALT), and the mucus overlying the epithelium. The intestinal mucus layer, host epithelial cells, GALT, and microbiome interact with each other forming a fragile and dynamic equilibrium, which is critically important for efficient functioning and absorption capacity of the digestive system. The physical (epithelial cells, intercellular tight junction, and mucus) and chemical (acidity, proteolytic enzymes, lysozymes, and antibacterial proteins) barriers play an important role in maintaining gut barrier function and preventing the microbial population from trans-locating and causing systemic immune activation. Besides acting as a physical barrier, the epithelial cells also secrete cytokines and chemokines that regulate chemotaxis of immune cells. Paneth cells located at the base of crypts of many vertebrate species, including poultry. It contains defensin rich granules that are released in response to bacterial induced inflammation (not during protozoal or fungal infection) via exocytosis. Three mucosal barrier factors help to maintain and restore the mucosal integrity of intestine; diamine oxidase, trefoil factor, and transforming growth factor-α. Occludin, claudin, and zona occludens-1 are the three tight junction proteins that maintain the paracellular barrier. Goblet cells in the GIT produce mucin, which also plays an important role in maintaining gut barrier function. Mucin production can be increased several bacteria, including Lactobacillus, which can help to improve the gut barrier as pathogenic microbes are impeded by the dense mucous layer. However, optimal gut health is not characterized by complete absence of pathogenic microbiota, rather an intestinal microbiome with a high microbial and functional diversity as summarized in Yeoman and White.
The gastrointestinal microbiota species composition and bacterial numbers vary depending on animal age, the gastrointestinal location and a variety of nutritional and environmental factors. The key components of gastrointestinal functionality factors are: diet, effective structure and function of the gastrointestinal barrier, host interaction with the gastrointestinal microbiota, effective digestion and absorption of feed and effective immune status. While the relationships between these areas is extremely complex, a multidisciplinary approach is needed to develop nutritional strategies that would allow farm animals to become more resilient to the environmental and physiological challenges that they will have to endure during their productive career. As the demand of animal products from the rapidly growing world human population is ever growing, there remedy is to establish a multidisciplinary approach to increase animal health, welfare and performance.
Diaz Carrasco, et al. reported the main factors as shape the process of gut microbiota acquisition and maturation, their interactions with chicken immune homeostasis, and the outcome of these interactions on intestinal health and productivity. The symbiotic relationship between ruminants and rumen microorganisms is paramount to the conversion of lowquality feed to high-quality end products. As the livestock industry faces a challenge to produce more pounds of meat and milk to meet the demands of the growing human population, continued improvements in production efficiency are essential. It has long been recognized that rumen microbes play an essential role in host health and performance. The microbial composition in the rumen changes with host growth and development before stabilization at weaning. The composition becomes relatively stable with maturity, indicating that the potential to influence the composition of the rumen microbiome, and ultimately host animal performance, occurs earlier in life (e.g., before birth, at/near birth, before maturity). Contributions of maternal, genetic, and environmental factors on rumen microbiome colonization and establishment, it may be possible to influence the early microbiome to favor improved host lifetime health and performance. The identification of feed efficient animals may be the single most impactful advancement towards long-term livestock sustainability and the promise of feeding the world animal products.
Diet: Specific foods and dietary patterns can all influence the abundance of different types of bacteria in the gut, which in turn can affect health. The interplay between the commensal microbiota and the mammalian immune system development and function includes multifold interactions in homeostasis and disease. The microbiome plays critical roles in the training and development of major components of the host’s innate and adaptive immune system, while the immune system orchestrates the maintenance of key features of host-microbe symbiosis. In a genetically susceptible host, imbalances in microbiota-immunity interactions under defined environmental contexts are believed to contribute to the pathogenesis of a multitude of immunemediated disorders. Mammals’ age, diet, health and pathological status might be influenced by the percentage of individual/ various microbial groups. Normally, herbivores, carnivores and omnivores are respectively characterized by a high, low and intermediate number of bacterial phyla.
Dietary fiber is an inevitable component in pig diets. In nonruminants, it may influence many physiological processes in the Gastrointestinal Tract (GIT) such as transit time as well as nutrient digestion and absorption. Moreover, dietary fiber is also the main substrate of intestinal bacteria. The bacterial community structure is largely susceptible to changes in the fiber content of a pig's diet. Indeed, bacterial composition in the lower GIT will adapt to the supply of high levels of dietary fiber by increased growth of bacteria with cellulolytic, pectinolytic and hemicellulolytic activities such as Ruminococcus spp., Bacteroides spp. and Clostridium spp. Furthermore, there is growing evidence for growth promotion of beneficial bacteria, such as lactobacilli and bifidobacteria, by certain types of dietary fiber in the small intestine of pigs. Studies in rats have shown that both Phosphorus (P) and Calcium (Ca) play an important role in the fermentative activity and growth of the intestinal microbiota. This can be attributed to the significance of P for the bacterial cell metabolism and to the buffering functions of Ca-phosphate in intestinal digesta. Moreover, under P deficient conditions, ruminal NDF degradation as well as VFA and bacterial ATP production are reduced. Similar studies in pigs are scarce but there is some evidence that dietary fiber may influence the ileal and fecal P digestibility as well as P disappearance in the large intestine, probably due to microbial P requirement for fermentation. On the other hand, fermentation of dietary fiber may improve the availability of minerals such as P and Ca which can be subsequently absorbed and/or utilized by the microbiota of the pig's large intestine.
The goals of raising calves to weaning age are optimizing growth and minimizing health problems. We do this by understanding the digestive system, immune system, nutrient needs, and feed options. Proper feeding and care of young calves is the first step in raising healthy, productive replacement animals to enter your milking herd. Feed four quarts of high-quality colostrum within the first eight hours to provide calves with essential nutrients and antibodies. Match milk replacer to growth and weaning age goals to meet calves' needs and to balance feed costs and animal performance. Offer a palatable calf starter by three days of age to stimulate rumen development and allow weaning by four to six weeks of age. Remove uneaten starter daily to maintain freshness. Finally, remember that nutrition is not the only factor affecting calf health and growth. Provide calves with clean, dry, draft free housing that protects them from harsh sun in the summer and cold winds in the winter. From three days of age, make fresh, clean, free choice water available. Work with your veterinarian to ensure that calves receive adequate vaccination and to develop treatment protocols for sick calves.
Ruminants are prone to many nutritional and dietary transitions throughout life. Even as pre-ruminants, these animals shift from ingesting milk only to ingesting some solids, and depending on management practices, they could also be fed concentrate starter pellets. Once the rumen is functional, ruminants continue to experience dietary shifts; some shifts are imposed by design, whereas others are a result of environmental and seasonal shifts that alter the availability and quality of feedstuffs. Weaning and the transition to feedlot for finishing are prominent periods that require microbiome shifts to allow continued, feasible performance of the host.
Host effects: Different animal species have different characteristics of their digestive system. It is largely related to their unique anatomical structure and diet type. Therefore, different input and process result in different output, for example the amount of soluble carbohydrate or fiber reaching the large intestine varies among different species. All of these inevitably give rise to a unique Gastrointestinal Tract (GIT) microbiota for each species. Among all digestive types, monastic herbivorous animals have been reported susceptible to the change of microbial communities in their digestive tracts.
Effective functionality of the Gastrointestinal Tract (GIT) and its health, are important factors in determining animal performance. The key components of gastrointestinal are: diet, effective structure and function of the gastrointestinal barrier, host interaction with the gastrointestinal microbiota, effective digestion and absorption of feed and effective immune status. While the relationships between these areas is extremely complex, a multidisciplinary approach is needed to develop nutritional strategies that would allow farm animals to become more resilient to the environmental and physiological challenges that they will have to endure during their productive career that can be used to establish a multidisciplinary approach to increase animal health, welfare and performance (Figure 3).
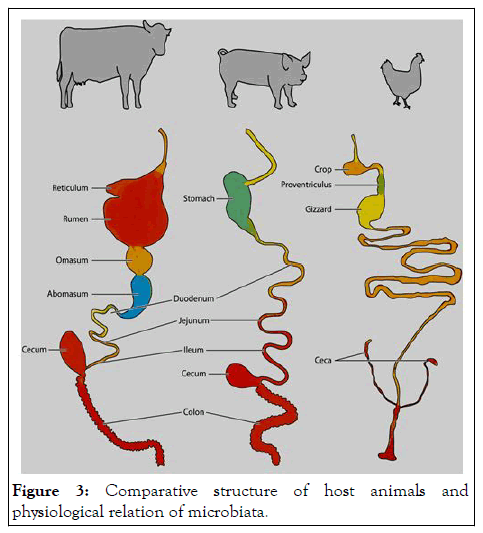
Figure 3: Comparative structure of host animals and physiological relation of microbiata.
The gut microbiota of mongastric: Gastrointestinal microbiota has significant impact on the nutrition and health of mongastric herbivores animals including donkey. Donkey has a rich, diverse and multi-functional microbiota along the GIT. In general, the richness and diversity of the microbiota are much higher in the hindgut relative to that in the foregut; at phylum level, the Firmicutes is dominant in the foregut while both Firmicutes and Bacteroides are abundant in the hindgut; at the genus level, Lactobacillus was dominant in the foregut while Streptococcus was more dominant in the hindgut. PICRUSt analysis showed that varying microbiota along the GIT is functionally compatible with the corresponding physiological function of different GIT sites. For example, the microbes in the foregut are more active at carbohydrate metabolism, and in the hindgut are more active at amino acid metabolism. The GIT of poultry consists of the esophagus, crop, proventriculus, gizzard, duodenum, jejunum, ileum, cecum, colon, and cloaca. Poultry GIT is much shorter as compared to other mammals relative to their body length. Thus, microbiota that grows in such a small GIT with relatively low transit time requires unique adaptations to adhere to the mucosal wall and to proliferate. The ceca have lower passage rate and are favorable to diverse groups of bacteria, which affect nutrient utilization and overall health of poultry.
Like all homoeothermic animals, chickens have a complex intestinal microbiota, the composition and metabolism which vary between intestinal compartments with highly different physicochemical microenvironments. The main factors that drive the fitness and colonization efficiency of the microbes are the availability of suitable growth substrates, prevailing pH and redox potential and the antibacterial secretions of the host in a specific intestinal section. The availability of easy growth substrates for bacteria decreases on moving down the gastrointestinal tract. That is why bacteria in the lower intestine are often specialists in utilizing feed components that are resistant to the endogenous digestive system of the host, e.g. non-starch polysaccharides, resistant starch or resistant protein. The proximal GIT (crop, proventriculus, and gizzard) is characterized by low pH, which strongly selects bacteria and limits the growth of many species. Microbiota composition of jejunum and caecum is highly different.
The different sections of the broiler chicken intestinal tract are inhabited by specialist microbiota adapted to the physicochemical conditions, host physiology and available nutrients of the specific habitat. The small intestine is dominated by lactic acid bacteria which have complex nutrient requirements resembling those of the chicken host itself. Lactobacilli are unable to synthesize amino acids for their anabolism and are therefore highly dependent on amino acid availability in the growth environment. Thus, in the small intestine there is competition for amino acids between the microbiota and the chicken host. If the protein is highly digestible and amino acids are largely absorbed in the upper small intestine, where bacterial growth is suppressed, the proportion captured by the host may be higher. Exogenous enzymes which promote protein digestion are also likely to provide a competitive advantage to the chicken, offering less growth potential for amino acid dependent bacteria. Protein escaping the ileum comprises resistant protein of dietary origin, protein assimilated to intestinal bacteria and endogenous protein synthesized and secreted by the host, the latter synthesized in host tissues from dietary amino acids and thus representing true endogenous protein. Activities of small intestinal bacteria affect the size of the microbial protein fraction and also the production of endogenous proteins originating from mucin, epithelial cells and antibodies.
The bovine rumen microbiome: The rumen microbial ecosystem is diverse and complex, consisting of microorganisms working symbiotically to break down feedstuffs consumed by ruminant animals. The microbiome controls the production efficiency of the animal, with certain pathways (those associated with methane production) resulting in energy loss in the animal. The microbiome also affects endproduct quality (milk and meat) but also contributes to environmental pollution.
Ruminants, through the action of their microbiota, can utilize components that the human body cannot break down, namely lignocellulose. Lignocellulose is the most abundant carbon polymer on the planet, with the rumen having a central role in releasing this vast energy store. The rumen ultimately uses lignocellulose to make products (i.e. milk and meat) that are then available to humans to consume as a nutrient dense food source. The interaction between the host and microbes in the rumen is synergistic, in that the host provides heat, moisture and food, while the microorganisms produce protein and byproducts of digestion, such as VFAs, for use by the host. The microbes are lastly degraded by the host with microbes utilized for their protein, lipid and starch content.
Bacteria are the most abundant microbes in the foregut of ruminant animals, with approximately 1010–1011 cells/ml and over 200 species. The composition of the bacteria found in the rumen is dictated by a number of factors including preference for certain substrates (i.e. diet), energy requirements, and resistance to certain metabolic end products that may be toxic to some species. Rumen bacterial composition is mainly Gram negative when animals are being fed high forage diets, with more Gram positive bacteria, such as Lactobacillus, present in animals fed high grain diets, with ruminal pH levels dropping after the consumption of easily fermented carbohydrates. The rumen can be viewed as an anaerobic and methanogenic fermentation chamber that contains microorganisms that have the ability to utilize and increase the productivity of cellulolytic feeds (i.e. straw, hay, silage and grass). There are considerable benefits associated with understanding rumen function, as rumen dynamics are almost solely responsible for providing nutrients to the host animal. As a result of fermenting feedstuffs, Carbon dioxide (CO2) and Hydrogen (H2), which are the main electron acceptors and donors of the ecosystem, are produced in the rumen. There are three intersecting micro-environments found in the rumen that contain these microbes; the liquid phase making up 25% of the microbial mass, the solid phase making up 70% of the microbial mass, and the rumen epithelial cells and protozoa, containing 5% of the microbial mass. The pH of the rumen is kept relatively constant, typically 6–7, but may vary depending on diet. Such variations can result in a change in the microbial populations, and the levels of VFAs produced by them. These fatty acids are of interest as some, such as propionate and butyrate, can be absorbed across the gut wall to serve as an energy source for the ruminant. Buffering of the rumen to maintain a relatively constant pH is facilitated by the large quantity of saliva produced by the ruminant, which is high in sodium and potassium bicarbonate and urea. The saliva is swallowed into the rumen and then absorbed through the rumen walls. Further buffering is provided by ammonia produced during fermentation, which can then be used for microbial growth in the rumen.
Protein available to ruminants is supplied by both microbial and dietary sources. Metabolizable protein (MP is the true protein which is absorbed by the intestine and supplied by both microbial protein and protein which escapes degradation in the rumen; the protein which is available to the animal for maintenance, growth, fetal growth during gestation, and milk production. In India, ruminant rations are still balanced for digestible CP and total digestible nutrients for protein and energy requirements, respectively. Traditional feed analysis methods such as proximate analysis and detergent analysis consider feed protein as a single unit and do not take into account of the degradation processes that occur in rumen and passage rates of feed fractions from rumen to intestine. Therefore, the protein requirement of ruminants should include not only the dietary protein source, but also the microbial CP from rumen. The MP systems consider both the factors, thus predict the protein availability more accurately and precisely. This system is aptly designed to represent the extent of protein degradation in the rumen and the synthesis of microbial protein as variable functions. Feed protein fractions, i.e., rumen degradable protein and rumen degradable protein play vital roles in meeting protein requirements of rumen microbes and host animal, respectively. Overall, there is a complex network of rumen microorganisms that interact and compete for substrates resulting in a critical balance of end products to provide energy for microbial growth, further fermentation, and beneficial end products for the host.
The gut microbial ecology is very vast contributing essential part of the host life in terms of feed digestion, fermentation and absorption and by far securing the survival by boosting immunity. Since the GIT enable the host to better productivity due to improved feed intake and feed conversion efficiency, there would be minimal environmental pollution due to GHG like CH4 and CO2 emission and overall health and economic advantage of the alarming growing human population, demanding livestock. The key components of gastrointestinal functionality are: Diet, effective structure and function of the gastrointestinal barrier, host interaction with the gastrointestinal microbiota, effective digestion and absorption of feed and effective immune status. However, effective functionality of the GIT and its health, are important factors in determining animal performance, since there are several complex mechanisms are involved in the regulation of GIT functionality and health, therefore it is crucial to deepen our knowledge of these interactions so that strategies for the modulation of GIT functionality and health, in context of improved animal performance, can be developed. While the relationships between these areas is extremely complex, a multidisciplinary approach is needed to develop nutritional strategies that would allow farm animals to become more resilient to the environmental and physiological challenges that they will have to endure during their productive career.
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
[Crossref] [Google scholar] [Indexed]
Citation: Negash A (2022) Gut Microbiota Ecology Role in animal Nutrition and Health Performance. J Clin Microbiol Antimicrob. 6:001.
Received: 15-Jun-2022, Manuscript No. JCMA-22-17950; Editor assigned: 16-Jun-2022, Pre QC No. JCMA-22-17950(PQ); Reviewed: 30-Jun-2022, QC No. JCMA-22-17950; Revised: 16-Sep-2022, Manuscript No. JCMA-22-17950(R); Published: 23-Sep-2022 , DOI: 10.35248/JCMA. 22.6.001
Copyright: © 2022 Negash A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.