
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Review Article - (2024)Volume 15, Issue 5
The choice of the right hemodynamic monitoring system for those patients at increased risk for anestesia is crucial. The interaction of general anesthesia and surgical stress represent the main special problem and the leading cause for postoperative morbidity and mortality. Anesthesiologists, firstly, should recognize the patient at high risk, matching for the type of surgical procedure and choose the proper hemodynamic monitoring device. In this review we summarize the benefit of a drawn path which begins before surgery and discuss the available evidence supporting the efficacy and safety of an individualized hemodynamic approach for major non-cardiac surgery.
Anestesia; Surgical stress; Non-cardiac surgery; Patients
Over the years surgical procedures have become increasingly complex and lasting. When elderly patients, with a higher probabilty to have comorbidities, are scheduled for these operations, the rate of postoperative complications and death are greatly increased. So, a first step is the recognition of the highrisk surgical patients and stratification. Cardiac disordes, as well pulmonary and metabolic syndromes, are associated with a higher frequency of adverse events and death after surgery. Bland was among the first to adopt a combination of a hemodynamic and oxygen transport parameters during surgery and find a correlation with poor outcome. As matter of this, identification and treatment of further correctable problems (i.e. anemia and electrolyte disturbances) is crucial to the successful conduct of anesthesia and surgery [1].
Stratification of patients
Preoperative evaluation remains a key step in assessment of those patients at high risk of cardiac adverse events and which could account for 80% of overall deaths. Accurate cardiac risk prediction can aid physicians in the making decision process, about the appropriateness of the procedure and guiding intraoperative management as well to identify patients who require postoperative adequate monitoring.
Tarhan and collegues was the first who showed that the delay of surgery could reduce the rate of postoperative myocardial ischemia from 37% to about 5% in those patients with recent myocardial infarction. Therefore to recognize this subgroup of patients for preoperative optimization, together to an intraoperative individualized management, can potentially improve outcomes. Different preoperative scores exist and available to anesthesiologists for surgical risk stratification; the most commonly used are the American Society of Anesthesiologists’ Physical Status (ASA-PS) classificatiton, Revised Cardiac Risk Index (RCRI) and American College of Surgeons’ National Surgical Quality Improvement Program Risk Calculators (ACSNSQIP) [2].
ASA-PS has been shown to have independent association with postoperative morbidity and mortality but limited to lower rather than higher mortality settings though the class assignment is independent of the surgical procedure and is based on the patients’ overall health status. The RCRI is widely used to predict Major Adverse Cardiovascular Events (MACE) in the context of non-cardiac surgery, but with limited predictive performance in the vascular surgical population. High-risk surgery, ischemic heart disease, congestive heart failure, cerebrovascular disease, preoperative treatment with insulin and preoperative serum creatinine above 2 mg/dl are the six independent predictors included in RCRI score.
ACS-NSQIP is freely available online calculator which serves as a handy one-stop shop for postoperative risk assessment including also patient-centered outcome variables such as readmission rate and non-home discharge [3].
According the recent American College of Cardiology and American Heart Association guidelines, this score provides a good estimate of surgery-specific risk of MACE or death. Two limits emerged for this last score: Lack of an external validation outside the United States by multicenter studies and the requirememt of internet connectivity. Once the patient at high-risk is identified a goal-directed therapy is strongly recommended, as the benefits will be evident. But a guided hemodynamic protocol could be useful for low-risk patients with a consistent tumor load, when a very extensive surgery is proposede. Hence matching monitoring needs to patient and surgical risk might help anesthesiologists (Figure 1) [4].
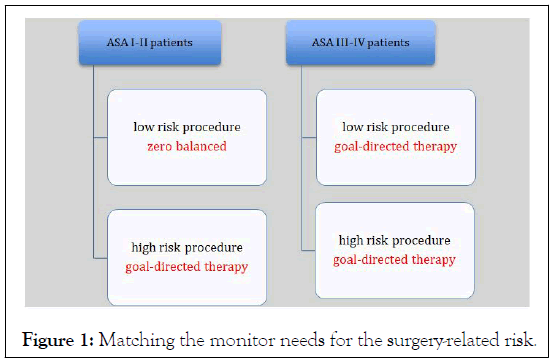
Figure 1: Matching the monitor needs for the surgery-related risk.
Targets and tools
Impairment of tissue perfusion and cellular oxygen deliver are probably the leading cause of perioperative complications and poor outcomes. The peripheral arterial oxygen saturation (SpO2), the blood pressure (both invasive or not) and heart rate are important and simple diagnostic tools but unsuitable for discriminate a stable from unstable patient. Despite some limitations, these parameters can help to identify a functional but nonspecific change (i.e. tachycardia during a tilt-up test; from Trendelenburg to supine positioning; high versus low or zero positive end-expiratory pressure). In a pre-shock status this basic hemodynamic parameters are still in a normal value because of compensatory reflex mechanisms to support blood flow and organ perfusion [5]. Hence an unanswered question in hemodynamic support is the optimal level of tissutal perfusion. To prevent tissue hypoperfusion and disoxya, target-oriented protocols have proven to ameliorate outcomes. More than 20 years ago Shoemaker found that in 708 high risk surgical patients, survivors had greater postoperative increase in Cardiac Index (CI), Delivery of Oxygen (DO2), Oxygen Consumption (VO2) and other flow-related variables than did the nonsurvivors. Afterwards, a large number of studies have been conducted to clarify the real need of measuring oxygen transport parameters; why cardiac output is measured? Clearly, it represents the physiological pillar next to the oxygen content (CaO2, the arterial quantity of O2) in the description of the DO2. Everyone must remember the following formula: DO2=10 × CO × CaO2 where this last depends mainly on the hemoglobin value and SpO2. And CO is equal the product of Stroke Volume (SV) and Heart Rate (HR) as follows: CO= SV × MAP. Hence, CO can be optimized trhough a proper fluid management aiming at SV optimization; nay fluid administration should be guided by CO measurement. The concept of preload dependence/independence describes the effect of fluid administration on CO; responders are those patients in the steep portion of Frank-Starling curve in which fluid loading move them towards the flat portion of the curve (Figure 2) [6].
Figure 2: Frank-Starling curve and the hemodynamic optimization target.
Because of the absence of non-invasive technologies to monitor blood flow, Pulmonary Artery Catheter (PAC) was commonly used in the past. Its invasiveness, however, limited the application to particular patient subgroups (cardiac surgery, liver transplantation and ICU patients). The technique of arterial waveform analysis is considered to be minimally invasive, providing a real time and continuous data [7]. The way to keep a normal CO, if hemoglobin and SpO2 are in the range, is to give fluids. To optimise fluid administration, several authors have investigated the association between the CO-target fluid therapy by the use of Doppler Oesophageal (TED) probes and postoperative endpoints. They demonstrated the superiority of a goal fluid therapy compared to conventional parameters such as arterial blood pressure, central venosu pressure, heart rate and urine output. TED has proven to be in agreement but not interchangeable with the gold standard for CO measurement, i.e., PAC, with a low mean bias. Doppler probes put into the aorta and Transesophageal Echocaridogtaphy (TEE) can provide immediate point-of-care evaluation of sudden hemodynamic fluctuations during surgery and in addition to CO measurement, TEE allows an accurate estimation of Left Ventricular (LV) contractility, as well as testing the fluid responsiveness.
In absence of a guide hemodynamic protocol, physicians should manage fluids by a restricted or liberal regimen but both inappropriate to prevent any deleterious consequence of under or overloading [8].
To understand when fluids should be administered the Frank-Starling curve is necessary; to keep patients at the flat portion of that curve means that a further fluid challenge is not requested. A U-shaped curve describe the relationship between hypovolemia, overhydration and the morbidity rate. In mechanically ventilated patients, according to the Frank-Starling mechanism, cyclic changes in vena cava, pulmonary artery and aorta blood flow generating such variations in SV and arterial Pulse Pressure (PP). These dynamic changes due to the heart-lungs interaction produce dynamic variables (Stroke Volume Variations, SVV and Pulse Pressure Variations, PPV) which can accurately predict fluid responsiveness, greater than traditional static parameters (i.e., Central Venous Pressure, CVP). When volume optimization is achieved by using this so-called dynamic indices, hemodynamic stability as well reduced postoperative complications are obtained. Interestingly a recent metanalysis found that the combination of more goals was associated with a significant improvement in postoperative clinical outcomes [9].
Despite the growing number of clinical studies demonstrating the efficacy of GDT, a recent survey showed that a minority of anesthesists use a target fluid therapy in high-risk surgical patients. Anyway, some limitations exist: A consistent heterogeneity among protocols and institutional practice emerged: Different targets, different interventions and mostly, different surgical setting as well the small size. On the other hand, it is not yet clear what is the right threshold for SVV and PPV which discriminate the fluid-responder from a nonfluid-responder patient; probably a grey zone (from 10% to 15%) should be investigated [10]. Furthermore, accuracy and validation of these variables is related to some conditions: Sinusal cardiac rythm and mechanical ventilation with a tidal volume at least of 7 ml/kg. Besides, vascular tone as well high dose of vasoactive drugs, wich impact on LV filling and emptying, could alter SVV and PPV by altering the equilibrium between stressed and unstressed volume. Norepinephrine bolus or infusion lead to a recruitment of splanchnic blood, pushing it towards the heart, with a corresponding increase of venous return and the a reduction of SVV/PPV. Conversely, venodilators could produce a rise of SVV and PPV by a higher redistribution of circulating blood. Increasing volume could be obtained also by increasing pressure (i.e., CO=MAP-CVP/Vascular Resistance); at this point a further physiological property should be mentioned: Arterial elastance (Ea).
As shown in Figure 3 a patient could be fluid responder according a high level of SVV but fluid challenge may be appropriate only with highcompliance vessels. Dynamic Elastance (Eadyn), a functional approach to the assessment of arterial tone, has been proven to predict the hemodynamic response in MAP to fluid administration in hypotensive, preload-dependent patients. Then, additional informations could help anesthesiologists in the decision-making process facing a critical situation.
Since the aforementioned variables, commonly used during a hemodynamic protocol, cannot be used to rule out imbalances between oxygen supply and demand, metabolic parameters should be also considered to identify and propmtly treat an occult hypoperfusion [11]. Clearly, an arterial line plus a central venous catheter are usually positioned to manage highrisk surgical patients; hence central venous oxygen saturation (SvO2) and the arterial to venous carbon dioxide difference (PCO2-GAP) could be easily acquired. Low SvO2, as well high delta-PCO2, which reflects important changes in the O2 delivery/consumption relationship, are closely associated with increased postoperative complications. The optimal SvO2 value was 70.6% (sensitivity 72.9%, specificity 71.4%) for discrimination of patients who did and did not develop complications. Moreover, Robin and colleagues found that high-risk surgical patients with PCO2 values greater than 6 mmHg at admission in an Intensive Care Unit (ICU) showed higher rate of complications [12].
Figure 3: A pressure-volume curve demonstrating the possibility to correct volume by pressure; indeed vasopressor drugs able to increase cardiac output based on the vasomotor tone. If vessels are in a high-compliance status, fluid challenge could be inappropriate, at the same MAP and SVV values.
Finally, after classifying the patient as high-risk, anesthesiologists should plan a hemodynamic optimization protocol by choosing one or plus flow-related indices (dynamics better than statics) togheter to a metabolic parameter (SvO2, PCO2 gap, lactate value). This could guarantee a intraoperative hemodynamic stability and better results in terms of postoperative outcomes.
Action
If the real trigger for any intervention is the hemodynamic instability, anesthesiologists should keep in mind the three pillars of hemodynamic system: Preload, afterload and contractility (Figure 4). Fluids, inotropes and vasoconstrictors represent the basis of each protocol [13].
Figure 4: Hemodynamic pillars-targets and tools.
But before any hemodynamic intervention “Anesthesiologists should optimize the use of anesthetic drugs (i.e., alogenates, opioids etc.) by integrating additional information such the bispectral index”. An excessive depth of anesthesia could be deleterious and cause vasodilation and arerial hypotension.
If SVV or PPV have been selected as targets, a fluid challenge might be considered for value above 10%-15%; but if Eadyn is not available and MAP is stably below 65 mmHg (lasting at least 3 minutes), a norepinephrine infusion is requested to recruit the unstressed volume and possibly to correct the dynamic indices.
Afterwards, if SVV or PPV remain altered fluid administration will be the right intervention. As regard the type of solution, colloids or crystalloids have been used and the superiority of one rather than another has never been demonstrated. Most of studies have focused on the effects of different type of fluids in the setting of ICU, for critically ill patients.
For this subgroup of patients the chest trial documented a greater risk of developing renal dysfunction in the colloids group. On the other side, we can assume that patients underwent to elective noncardiac surgery have an intact vascular endothelial bed; indeed colloids administration for volume expansion during surgery seems to be safe. It may be inadvisable to use excessive amount of saline solution because of the greater risk of hyperchloremic acidosis. As inotrope drug the evidence support the use of dobutamine infusion to keep Cardiac Index (CI) above 2.5 L/min/m2. A recent review demonstrated that vasoactive drugs had significant positive effects in terms of complications and hospital stay for non-cardiac surgery.
Achieving and maintaining optimal or normal values of hemodynamic and metabolic parameters is the last step of this dynamic method. Using a monitoring system without a protocol or prefixed targets could not produce the beneficial expected results (Table 1). Hourly or whenever necessary, it should testing fluid responsiveness together to sample blood assessing early signs of tissue hypoperfusion.
|
|
|
| SVV | <10% |
| CI | >2.5 L/Min/m2 |
| MAP | >65 mmHg |
| SvO2 | >70% |
| CO2-GAP | <6 mmHg |
| LACTATES | <3 mmol/L |
Table 1: Hemodynamic and metabolic endpoints.
The decision regarding the extent of monitoring necessary in an individual and the time at which it should be commenced is guided primarily by the characteristics of the patient and the proposed surgical technique. Nowadays a conventional monitoring adopted for low-risk surgical patients involves recording of non-invasive blood pressure, heart rate by electrocardiographic trace and peripheral oxygen saturation. But if the patient and or the surgery-related risk increase then also an adequate hemodynamic monitoring should be used. Highrisk patients and operations could require a more advanced and invasive monitoring system and to establish a target is critical in order to ensure a perioperative hemodynamic stability. Hence the principal goal should be to avoid or treat hemodynamic instability.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Russo A (2024) Haemodynamic Monitoring: When and How-A Narrative Review and Proposal for a Method. J Anesth Clin Res. 15:1149.
Received: 13-Apr-2020, Manuscript No. JACR-24-3852; Editor assigned: 16-Apr-2020, Pre QC No. JACR-24-3852 (PQ); Reviewed: 30-Apr-2020, QC No. JACR-24-3852; Revised: 03-Jun-2024, Manuscript No. JACR-24-3852 (R); Published: 28-Oct-2024 , DOI: 10.35248/2155-6148.24.15.1160
Copyright: © 2024 Russo A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.