Journal of Phonetics & Audiology
Open Access
ISSN: 2471-9455
ISSN: 2471-9455
Research Article - (2021)Volume 7, Issue 2
Objective: The aim of this study is to evaluate the influence of Systemic Lupus Erythematosus (SLE) type, duration, severity as well a s prevalence on hearing loss on SLE patients.
Materials and methods: This study was conducted 98 SLE patients; 16 males and 82 females, and 20 healthy subjects; 5 males and 15 females served as controls. Pure Tone Audiometry (PTA), air and bone conduction threshold were done for all volunteers.
Results: PTA and air conduction threshold showed significant decrease in SLE group than controls (P<0.05), while bone conduction threshold shown significant increase in SLE group than controls (P<0.05). It was observed also that there is a significant relation between SLE severity and duration with Sensorineural Hearing Loss (SNHL).
Conclusion: The results of this study concluded that there is impact of hearing loss in SLE patients, it was observed that SLE severity and duration may affect the degree of hearing loss in SLE patients, it was observed that SLE severity and duration may affect the degree of hearing loss.
Lupus; Erythematosus; Choclea; SLE; SNHL; PTA; Glucocorticoid
Systemic lupus erythematosus is an autoimmune disease that involve multiorgan with incidence of up to 39 per 100,000 people in general [1]. The incidence of SLE is higher in women (82%–96%) than in men (4%–18%) [2] and it is three times more prevalent in Asians and Afric ans than Europeans. Onset is most prominent from 20 to 39 years of age [3].
It has been suggested that immunologic processes may involve inner ear [4,5]. It is also true for SLE too. Several studies have also shown the effect of SLE in the inner ear [1,6,7]. Kastaniousdakis et al. [8] and Sperling et al. [9] have reported that patients with SLE may complain from auditory symptoms. In the majority of cases, hearing loss is unilateral and associated with vestibular symptoms. The pathophysiology is unclear. Genetic, viral, traumatic, or toxic causes were previously discussed. Vascular or autoimmune etiology has been proposed in 10% of cases [10].
Furthermore, various studies proposed a number of mechanisms such as vasculitis processes or free radicals’ formation in steria vessels of choclea (similar to animal models of lupus), autoimmunity due to vasculitis, early presbycusis, drugs toxicity, micro-infarctions in capillaries and arterioles of temporal bone, thrombosis in the vessels of ear, and anti-phospholipid syndrome to deduce mechanism(s) of auditory involvement in SLE [11,12].
Different studies have reported diverse rates for prevalence of auditory disturbance in SLE. Auditory disturbance has been reported in 8-66 percent of patients with SLE [13,14]. In a study, hearing threshold had been declined in all frequencies except 2000 to 4000 Hertz in patients with SLE, while decline in hearing threshold has been more prominent at 2000 to 4000 Hertz; like an early presbycusis in another study [15].
The aim of this study was to evaluate the influence of SLE type, duration, severity as well as its prevalence of hearing loss in patients with SLE.
This cross-sectional survey design study was conducted on ninety-eight (98) patients diagnosed SLE according to SLICC criteria 2012 [10]. These patients represent the study group (1). Another group consisted of twenty (20) normal healthy adult subjects with normal hearing threshold according to ANSI were selected from relatives accompanying patients represent the control group. The age of both groups ranged from (20-40) years and represented both genders.
All patients and controls were recruited from Damietta, Al-Azhar University Hospital, Egypt from May 2018 to August 2019. They were selected randomly from patients attending the internal medicine and rheumatology clinics, Damietta Al-Azhar University Hospital, based on a single sequence of random assignments (simple randomization).
The protocol of this study was approved by the local ethics and research committee of Al-Azhar Faculty of Medicine (New Damietta), and informed consent was signed by all patients before inclusion in the study.
Normal otoscopic finding, normal hearing threshold and normal middle ear functions as evidenced by tympanometry and acoustic reflex were included, while patients with history of ear diseases (hearing loss, noise exposure, ototoxic drug intake, head trauma and others), and history of medical systemic diseases e.g., any rheumatic diseases, diabetes mellitus, hypertension, smoking, renal and cardiovascular diseases were excluded from the study.
Instrumentation
• Sound treated room locally made according to international
specifications.
• One channel intracoustic audiometer GSI (model AD229b),
calibrated according to ANSI standards.
• Impedance-meter interacoustics (model AT235).
Methods
Participants of both groups were subjected to the following:
• Full history taking to exclude history of systemic diseases,
noise exposure, ototoxic drug intake, and family history of
hearing impairment.
• Otological examination.
• Basic audiological evaluation, which includes the
following:Pure tone audiometry from (250–8000 Hz) for air
conduction, from (500–4000 Hz) for bone conduction in
octave steps. The air conduction stimulus was delivered via
supra-aural headphone model TDH 39P. The bone
conduction was delivered via bone conduction vibrator model
B71. Speech audiometry including:
Speech reception threshold (SRT) using Arabic spondee words.
Speech discrimination scores using Arabic phonetically balanced words (PB words).
Tympanometry which was done at pressure range from (+200 to –400 mm H2O).
Acoustic reflex threshold elicited ipsi-laterally and contra-laterally using frequency range of (500 up to 4000Hz).
• Laboratory studies that help in diagnosis and classification of
Systemic Lupus including (ESR, RF, ANA, anti-DNA anti
Smith, CBC, urine analysis, SGOT, SGPT, Anti cardiolipin,
lupus anti-coagulant).
• The disease severity index was determined for the patients
according to the standard criteria with systemic lupus disease
activity index (SLEDAI). Mild SLE: SLEDAI 0-5, moderate
SLE: SLEDAI 6-10, Severe SLE: >10.
Statistical analysis
Statistical analyses were completed using SPSS v23 statistical software (SPSS, Inc, Chicago, Illinois). Descriptive statistics (means, standard deviations, frequencies, and correlation coefficients) were calculated for all measures. To compare the two groups, a paired t-test was carried out to determine P values using the Pearson’s correlation test and a χ2 test and a one-sample t-test and Wilcoxon test performed when appropriate. In comparison between more than two groups (F) test by Analysis of Variance (ANOVA). P less than 0.05 was considered statistically significant.
The study included 98 SLE patients; 16 males and 82 females represent the patients’ group (1), their ages ranged from 20 to 40 years with a mean ± SD of 34.8 ± 5.69 years and 20 apparently healthy subjects; 5 males and 15 females represent the control group with age ranged from 20 to 40 years and mean ± SD of 33.8 ± 4.97 years. There is predominance of females in the patients’ group (P=0.012), as shown in Table 1.
| SLE group (1) N=98 | Control group (2) N=20 | Test of significance | ||||
|---|---|---|---|---|---|---|
| Gender | No. | % | No. | % | χ2 | P |
| Males | 16 | 16.33 | 5 | 25.0 | 0.632 | 0.002* |
| Females | 82 | 83.67 | 15 | 75.0 | 0.293 | 0.042* |
| Total | 98 | 100 | 20 | 100 | ||
Age (years):
|
Mean | ± SD | Mean | ± SD | t-test | P |
| 34.8 | 5.69 | 33.8 | 4.97 | 0.0015 | 0.869 | |
| Min | Max | Min | Max | |||
| 20 | 40 | 20 | 40 | χ2 | P | |
| Smoking: N (%) | 38 | 38.77 | 7 | 35 | 0.056 | 0.164 |
Table 1: Demographic characteristics of the studied groups.
χ2 =Chi square test, P>0.05= non-significant.
Measurement of air conduction hearing thresholds of both right and left ears shows significant decline in SLE compared to control subjects in all frequencies.
Pure tone audiometry shows also a statistically significant decrease in SLE compared to controls (P<0.05) as shown in Table 2.
| Frequency (Hertz) | SLE group (mean ± SD) | Control group (mean ± SD) | Significance | |
|---|---|---|---|---|
| Right ear: | T | P | ||
| 250 | 1.84 ± 3.47 | 5.96 ± 3.23 | 0.5241 | 0.004* |
| 500 | 1.91 ± 4.61 | 7.19 ± 2.54 | 0.6035 | 0.003* |
| 1000 | 2.24 ± 2.16 | 9.97 ± 5.24 | 0.8037 | 0.001* |
| 2000 | 2.83 ± 3.11 | 10.67 ± 6.18 | 0.8122 | 0.001* |
| 4000 | 3.12 ± 3.09 | 12.84 ± 6.41 | 0.9108 | 0.001* |
| 8000 | 3.69 ± 3.52 | 14.68 ± 8.44 | 0.9535 | 0.001* |
|
|
|
|
|
|
| 250 | 1.73 ± 4.75 | 6.11 ± 4.67 | 0.5512 | 0.004* |
| 500 | 2.19 ± 4.13 | 8.41 ± 7.45 | 0.6997 | 0.003* |
| 1000 | 2.95 ± 5.33 | 9.57 ± 7.18 | 0.7279 | 0.002* |
| 2000 | 4.35 ± 5.47 | 11.92 ± 8.12 | 0.7989 | 0.002* |
| 4000 | 4.48 ± 5.19 | 14.78 ± 6.48 | 0.9382 | 0.001* |
| 8000 | 4.79 ± 4.96 | 15.54 ± 9.21 | 0.9458 | 0.001* |
|
|
|
|
|
|
| Mean ± SD | 8.12 ± 2.34 | 10.73 ± 2.65 | 0.2674 | 0.011* |
| Range | 5.63–13.09 | 6.11–14.6 | ||
| Note: *P<0.05=significant, PTA: Pure Tone Audiometry | ||||
Table 2: Air conduction hearing thresholds in both groups.
Measurement of bone conduction hearing thresholds of both right and left ears shows significant elevation in SLE compared to control subjects in all frequencies and their mains shows (P<0.05) as shown in Table 3.
| Frequency (Hertz) | SLE group (mean ± SD) | Control group (mean ± SD) | Significance | |
|---|---|---|---|---|
| Right ear: | T | P | ||
| 250 | 4.72 ± 8.22 | 0.37 ± 3.64 | 0.5362 | 0.004* |
| 500 | 4.24 ± 4.98 | 1.19 ± 4.14 | 0.4564 | 0.005* |
| 1000 | 3.55 ± 3.62 | 2.11 ± 3.95 | 0.2392 | 0.012* |
| 2000 | 2.89 ± 4.41 | 1.67 ± 2.18 | 0.2084 | 0.014* |
| 4000 | 5.12 ± 3.26 | 2.24 ± 3.45 | 0.4391 | 0.005* |
| Mean ± SD | 3.51 ± 3.36 | 1.25 ± 2.64 | 0.4008 | 0.007* |
|
|
|
|
|
|
| 250 | 3.53 ± 5.15 | 1.22 ± 4.18 | 0.3769 | 0.008* |
| 500 | 4.11 ± 4.19 | 1.41 ± 7.45 | 0.4282 | 0.006* |
| 1000 | 4.21 ± 5.13 | 2.12 ± 6.68 | 0.3367 | 0.009* |
| 2000 | 4.25 ± 5.44 | 2.23 ± 6.12 | 0.3284 | 0.009* |
| 4000 | 4.44 ± 5.18 | 3.18 ± 7.05 | 0.2174 | 0.013* |
| Mean ± SD | 4.18 ± 4.94 | 2.13 ± 6.65 | 0.3311 | 0.009* |
| Note: *P<0.05=significant. | ||||
Table 3: Bone conduction hearing thresholds in both groups.
So, the prevalence of hearing loss in SLE patients by PTA, air conduction threshold, and bone conduction threshold were 27.6%, 26.7% and 33.7%, respectively, with an average of 32.7% as reported in Table 4.
| Prevalence of SNHL | Yes | No | Significance | |||
|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | P value | |
| PTA | 27 | 27.6 | 71 | 72.4 | 19.634 | 0.000* |
| Air conduction threshold | 36 | 36.7 | 62 | 63.3 | 11.713 | 0.000* |
| Bone conduction threshold | 33 | 33.7 | 65 | 66.3 | 14.249 | 0.000* |
| Average | 32 | 32.7 | 66 | 67.3 | 14.249 | 0.000* |
| Note: χ2=Chi square test, *P<0.05=significant | ||||||
Table 4: Incidence of SNHL in SLE patients (N=98).
Comparison between the Disease severity index (SLEDAI) and hearing loss in patients of SLE group shows a significant relation between hearing loss and severity of SLE disease (P=0.39) as shown in Table 5.
| Hearing loss | SLEDAI (0-5) | SLEDAI (6-10) | SLEDAI (>10) | F test | P value |
|---|---|---|---|---|---|
| Yes | 27 | 10 | 3 | 0.193 | 0.039* |
| No | 53 | 4 | 1 | ||
| Note: *P<0.05=significant. | |||||
Table 5: Comparison between the Disease severity index (SLEDAI) and hearing loss in patients of the study group (2).
Normal hearing levels ranged from 0 to 20 db, increase more than 20 db indicates SNHL; as the heating level increased there is increase in the degree of SNHL. In correlation between duration of SLE disease and SNHL, there is a strong positive correlation (r=0.6395, p <0.001) as shown in Figure 1.
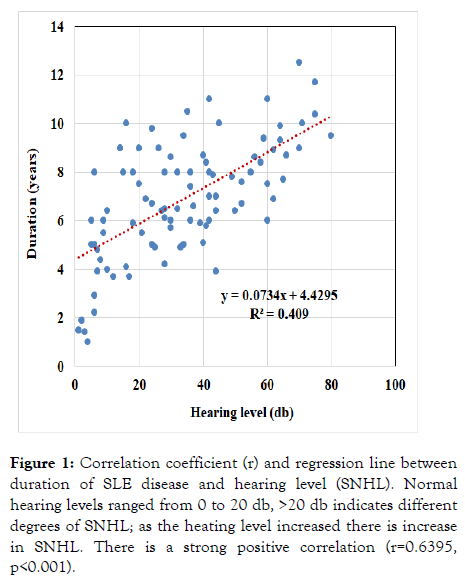
Figure 1: Correlation coefficient (r) and regression line between duration of SLE disease and hearing level (SNHL). Normal hearing levels ranged from 0 to 20 db, >20 db indicates different degrees of SNHL; as the heating level increased there is increase in SNHL. There is a strong positive correlation (r=0.6395, p<0.001).
Systemic lupus erythematosus affects mainly females which was presented in our study, this agreed by many studies [11-15]. It can affect inner ear by disrupting either the hearing or balance system. Affection of hearing can be anatomically categorized into conductive and sensorineural hearing loss [12].
Auditory disturbance has been reported in 8%-66% of patients with SLE [13,16,17]. Because of those differences in the reported related studies, the current study was conducted to evaluate auditory disturbance in patients with SLE and its correlation with severity and duration of the disease.
The current study reported progressive SNHL in all studied frequencies with different degrees (more observed in higher frequency) which was observed also by Roverano et al. [14] and Maciaszczyk et al. [15] described, bilateral, progressive SNHL in air conduction high frequencies. Khalidi et al. [6] described unilateral SNHL in mid and high frequencies (500 to 3000 Hz) associated with a 16% of word discrimination score recognized by speech audiometry.
In this study, air conduction hearing thresholds shows significant decline in SLE compared to control subjects in all frequencies. PTA shows also significant decrease in SLE group compared to controls (P<0.05). However, bone conduction hearing thresholds had a significant elevation in SLE compared to control subjects in all frequencies and their mains (P<0.05). This indicates that there is a great prevalence of hearing loss in SLE patients which was proved by PTA, air conduction threshold, and bone conduction threshold were 27.6%, 36.7% and 33.7%, respectively, with an average of 32.7%.
These results were slightly higher than Karatas et al. [13] who reported incidence of hearing loss in 21% of SLE patients, Kastanioudakis et al. [8] reported 21.5%, Abbasi et al. [18] reported incidence of 26.7%, Maciaszczyk and colleagues [15] reported 28.6% and lower than Roverano et al. [14] who reported an incidence of 66% hearing loss in SLE patients. All these studies and our study were opposed by the study of Polanski et al. [19], although they reported that SLE patients had more sensorineural loss than controls, no conductive hearing loss was detected in their study. They depend on clinical and serological lupus profile on their study.
It isn't conceivable to recognize the patients with hearing loss by the clinical or serological lupus profile. Gad and Abdulateef [20] studied the association between antiphospholipid antibody syndrome and hearing loss in children with SLE. In addition, many case studies associate sudden SNHL in SLE with the presence of autoantibodies.
We compared between the Disease severity index (SLEDAI) and hearing loss in patients of SLE group and found that there is a significant relation between hearing loss and severity of SLE disease (P=0.39). So, results of current study may imply that SNHL is directly related to the disease severity; that was opposed by Abbasi et al. [18], since SNHL is not related to the severity of the disease. Other mechanism should be taken in mind. More detailed study ought to be designed about the matter to explain the mechanism. If audiologic examination is conducted as soon as the diagnosis was done, more conclusive come about the mechanism and pathophysiology of SNHL may be achieved. Roverano et al. [14] and Maciaszczyk et al. [15] have found similar results.
SNHL was significantly correlated with duration of SLE disease (r=0.6395, p<0.001) in our study. This was inconsequent with the study of Maciaszczyk et al. [15] which had reported significant positive correlation between air conduction hearing loss and duration of SLE. Abbasi et al. [18], study was in contrary to these results. They found no association between duration of the disease and hearing threshold. It was emphasized that early detection of hearing loss in SLE is important for proper treatment [19], as the autoimmune etiology may respond to glucocorticoid and immunosuppressive treatment [6].
The results of this study concluded that there is impact of hearing loss in SLE patients, it was observed that SLE severity and duration may affect the degree of hearing loss. It is recommended that ear examination is mandatory in SLE patients as early as possible.
Citation: Ghanem SS, Alzokm SM (2023) Hearing Impairment in Patients with Systemic Lupus; Correlation with Duration and Severity of the Disease. J Phonet Audiol. 9:221.
Received: 29-Aug-2023, Manuscript No. JPAY-22-17718; Editor assigned: 01-Sep-2023, Pre QC No. JPAY-22-17718 (PQ); Reviewed: 15-Sep-2023, QC No. JPAY-22-17718; Revised: 22-Sep-2023, Manuscript No. JPAY-22-17718 (R); Published: 29-Sep-2023 , DOI: 10.35248/2471-9455.23.9.221
Copyright: © 2023 Ghanem SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.