Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2017) Volume 5, Issue 2
Background: This work assessed the distribution of Cd, Cr, Cu, Mn, Ni, Pb, and Zn in an important Brazilian fluvial section, which has never been evaluated before this study
Procedures: Along six samplings, total and bioavailable concentrations of these metals were quantified in water and sediments, respectively, by atomic spectrometry. Bioavailable metal concentrations were extracted from sediments by 0.1 molL-1 HCl, while total metal concentrations were released in water after acidic decomposition with 14 molL-1 HNO3. Sediments were submitted to analyses of X-ray diffractometry, thermogravimetry, infrared spectrophotometry, electronic scanning microscopy, granulometry, and density. Subsequently, adsorption tests were performed with those elements that tend to exist as cations in river water (Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+).
Results: Water pH (6.9 to 8.3) favored precipitation of Cr and Mn, explaining their undetectable concentrations in water. Although cadmium, copper, lead, nickel, and zinc are stabilized as cations at water pH and chemical oxygen demand (<0.5 mgL-1), total concentrations of these metals in water were undetectable. Sediments presented prevalence of quartz, sandy granulometry, maximum density of 2.8 gcm-3, and remarkable bioavailable concentrations of Cr (up to 778.4 ± 37.4 mg kg-1) and Mn (up to 230.9 ± 6.2 mg kg-1, which can be readily assimilated by benthic organisms. Bioavailable concentrations of Cd, Cu, Ni, Pb, and Zn in sediments were considerable small (≤ 43.4 ± 2.5 mgkg-1). Although sediments are limited for adsorbing Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ (≤ 1.49 mgg-1), these cations are adsorbed with low reversibility, being progressively accumulated and disseminated through the food chain over a long period of time.
Conclusions: This work improved the knowledge about the distribution of heavy metals in fluvial environments, as well as evaluated the risks of an eventual contamination of humans.
Keywords: Heavy metals; São Francisco river; Sediments; Adsorption; Mathematical model of Langmuir; Bioavailability
FAAS: Flame Atomic Absorption Spectrometry; ICPOES: Inductively Coupled Plasma Optical Emission Spectrometry
The São Francisco is one the most important rivers in Brazil and South America, with a length of 2,860 km and a watershed of 630,000 km2 [1]. It drains the areas of Minas Gerais (MG), Bahia (BA), Goiás (GO), Pernambuco (PE), Alagoas (AL), and Sergipe (SE), as well as part of the Federal District. The São Francisco River is of huge relevance for the Brazilian semiarid region (969,582 km2) that hosts 22 million of people and the Caatinga (84,453 km2), which is a biome exclusively occurring in Brazil [2].
In the lower São Francisco basin, this river separates Juazeiro, BA and Petrolina, PE, which are two cities with approximately 600,000 inhabitants and an economy based on cultivation of fruits (mostly grape and mango) and leather tanning. Besides its essential importance for the maintenance of all regional agricultural and industrial activities, the São Francisco is largely responsible for the supply of water for human consumption. Despite this, studies concerning the distribution and accumulation of heavy metals in sediments and water of this important Brazilian fluvial section have never been performed before this study. This lack of information continues, since no work has been published since February 2013, when the present work was finished. Ultimately, it means that the potential risks of human contamination by heavy metals through the consumption of contaminated aquatic organisms and water are still completely unknown in the assessed area.
In this work, the following heavy metals were selected for evaluation: trivalent Cr, Cd, Pb, Mn, Ni, and Zn. Trivalent Cr was chosen due to its use in the leather tanning industries, which are widely distributed in the cities of Juazeiro, BA and Petrolina, PE. Moreover, when in large excesses in a food chain, trivalent Cr can reach humans and cause stomach cancer [3]. Cd and Pb were evaluated because these elements are very toxic to humans even if small amounts are considered. Of the many human diseases that can be caused by Cd and Pb, infertility, leukemia, bone weakness, and psychiatric disorders should be focused on [4]. Although Cu, Mn, Ni, and Zn are essential elements, they can also exhibit toxic effects to humans if certain limits are exceeded. Excesses of Cu can promote blindness, while large ingestions of Mn manifest neurological damages. On the other hand, high exposure to Ni and Zn can be responsible for carcinogenicity [5,6]. Besides the potential risks concerning human health, Cu, Mn, Ni and Zn have widespread use in diverse types of industries and agricultural activities [4].
Thus, this work aimed at assessing the concentrations of Cd, Cr, Cu, Pb, Mn, Ni, and Zn in fluvial sediments between Juazeiro, BA and Petrolina, PE, as well as to correlate these concentrations with water chemical conditions (pH, chemical oxygen demand, and total concentrations of the cited metals) and sediment structural characteristics. This integrated approach is intended to evaluate the risks of dissemination of Cd, Cr, Cu, Mn, Ni, Pb, and Zn through the food chain with the consequent possibility of reaching humans.
Equipment and reactants
The following instruments were used: Gehaka Master P&D (Brazil) water purification unit, Quimis Q225M (Brazil) horizontal table, IKA basic A11 blade mill (Germany), Tecnal Tec-3MP potentiometer (Brazil), GBC SensAA flame atomic absorption spectrophotometer (Malaysia), Varian Vista-PRO inductively coupled plasma optical emission spectrometer (Australia), Rigaku X’Pert Pro PW 3040/60 X-ray diffractometer (Japan), TA Instruments TGA 2950 thermogravimetric analyser (USA), Perkin-Elmer Spectrum 100 infrared spectrophotometer (USA), and Jeol JSM-6610LV electron scanning microscope (Japan).
Single stock standard solutions of metals at 1,000 mgL-1 (SCP Science, USA) were used to calibrate the spectrometers and prepare all solutions used in the retention tests. 14 molL-1 HNO3 (Merck, Germany) and 0.1 molL-1 HCl (Vetec, Brazil) were used to perform total decomposition of water and extraction of bioavailable metals from sediments, respectively. All solutions were prepared with ultrapure water (18.2Ω cm-1).
Experimental part
Sampling points and procedures
Geographical locations of Juazeiro (BA) and Petrolina (PE) are indicated in Figure 1.
During six samplings, sediments and water were periodically taken at the six points indicated in Figure 2 in order to determine bioavailable and total contents of metals, respectively. Thus in all, 36 sediment samples and 36 water samples were analysed. These periodic samplings of sediments and water were made on 10/17/2011 (first), 12/05/2011 (second), 04/24/2012 (third), 05/29/2012 (fourth), 09/20/2012 (fifth), and 02/05/2013 (sixth). Chemical oxygen demand and pH were also measured in the 36 water samples. The sampling points indicated in Figure 2 are located near the following places: the campus of Bahia State University (point 1), urban riverbanks (points 2 and 3), a fluvial island (point 4), and leather tanneries (points 5 and 6).
Sediments, which were submitted to structural analyses, were collected at the points 1 to 9, indicated in Figure 3. For this purpose, sediments were collected only once, so that structural analyses were performed with nine sediment samples. The sediments collected at point 1 were also used to perform the retention tests with metallic cations. These sampling points are located near the following places: urban riverbanks (points 1, 2, 6 and 9), a fluvial island (point 3), leather tanneries (points 4 and 7), a rural property (point 5), and a sailing club (point 8).
Table 1 lists geographical coordinates of the sampling points shown in Figures 2 and 3.
| Sampling points | Coordinates | |
|---|---|---|
| Figure 2 | Figure 3 | |
| 1 | S 09° 24'51.5"WO 40° 29'10.8" | S 09° 24'41.6" W 040° 29' 38.4" |
| 2 | S 09° 24'41.5"WO 40° 29'36.7" | S 09° 24' 32.0" W 040° 30' 50.2" |
| 3 | S 09° 24'35.3" WO 40° 30'12.6" | S 09° 24' 22.9" W 040° 30' 18.6" |
| 4 | S 09° 24'19.3" WO 40° 30'18.2" | S 09° 25' 17.2" W 040° 28' 27.7" |
| 5 | S 09° 24'17.6"WO 40° 28'01.7" | S 09° 23' 40.5" W 040° 26' 58.0" |
| 6 | S 09° 25'15.4"WO 40° 28'31.6" | S 09° 22' 47.2"W 040° 26' 55.8" |
| 7 | - | S 09° 24'20.1" W 040° 28' 05.7" |
| 8 | - | S 09° 24'26.5" W 040° 29' 09.5" |
| 9 | - | S 09° 24'18.2" W 040° 31' 26.6" |
Table 1: Geographical coordinates of the sampling points indicated in Figure 2 and Figure 3.
All marginal and superficial samples of sediments (depth of 5 cm) and water (depth of 10 cm) were collected and preserved by following well-established procedures [7].
Analytical procedures
Periodic analyses of sediment and water samples
For periodic quantifications of bioavailable metals in sediments, the samples were dried in an oven at 100° C for 72 h, passed through a 10-mesh sieve, crushed in a blade mill, and sieved again at 270-mesh (nominal sieve opening of 0.053 mm). This procedure was performed to ensure a high sample homogenization, as well as precise and accurate results [8].
Portions of one gram of sediments were stirred at 200 rpm and 25°C for 2 h with 25.00 ml of 0.1 molL-1 HCl [9]. The supernatants were filtered and analyzed by flame atomic absorption spectrometry (FAAS) to quantify the bioavailable concentrations of Cd, Cu, Mn, Ni, Pb, and Zn, and by inductively coupled plasma optical emission spectrometry (ICP OES) to determine the bioavailable concentrations of Cr.
All water samples were pre-concentrated 10X by evaporation and submitted to acidic decomposition with 14 molL-1 HNO3 [10] to subsequent quantifications of total concentrations of Cd, Cu, Pb, Mn, Ni, and Zn by FAAS. Total Cr concentrations were quantified by ICP OES. Other water samples were also collected (Figure 2) to measure pH by means of a potentiometer. Water samples were also submitted to analyses of chemical oxygen demand [10] to estimate their contents of dissolved organic matter.
All analyses by FAAS were made by using air-acetylene flame (fluxes of air and acetylene of 8.0 and 2.0 L min-1, respectively) and background correction with a deuterium lamp. Hallow cathode lamps were operated in the following wavelengths (nm): 228.8 (Cd), 324.8 (Cu), 279.5 (Mn), 232.0 (Ni), 283.3 (Pb), and 213.9 (Zn). For quantification of Cr by ICP OES at 426.2 nm, the equipment was operated with a power of 1300 W and 40 MHz radiofrequency. All spectrometers were calibrated by means of daily calibration curves, which were built from successive dilutions of stock solutions of each metal at 1,000 mg L-1.
Structural analyses of the sediments
Before analyses of thermogravimetry, X-ray diffractrometry, scanning electron microscopy, granulometric composition, and density, sediments were dried in an oven at 100°C for 72 hours, and passed through a 10-mesh sieve (nominal sieve opening of 2.0 mm) to remove large fragments of plants and rocks. In this way, the natural granulometric distribution of sand (diameters from 2.0 to 0.5 mm), silt (diameters from 0.5 to 0.02 mm) and clay (diameters<0.02 mm) of these sediments was kept in the analyses described below.
The analyses of thermogravimetry were performed by heating samples from room temperature to 1,000°C (at 10°C min-1) in the presence of synthetic air. For the X-ray analysis, the samples were exposed to radiation (Co-Kαλ, =1.79026 Å) with 2θ angles varying from 5 to 50°. The applied current and voltage were 30 mA and 40 kV, respectively. Analyses by infrared spectrophotometry were performed using Kbr discs to prepare the sediment samples. The spectral range varied from 4,000 to 400 cm-1. For electron scanning microscopy, sediments were covered with a thin layer of gold and an acceleration voltage of 20 kV was applied. The peaks were associated to specific minerals following recommended procedures [11].
The granulometric compositions of sediments were determined by considering the vertical displacement time of clays (Stokes’s law) after addition of chemical dispersants [12].
To determine the density of sediments, 20 g of them were added to 50.00 ml volumetric flasks, whose volumes were subsequently filled with 95% (v/v) ethanol. The volumes of 95% (v/v) ethanol were recorded with a burette and the values of density were calculated by dividing the sediment mass (20 g) by the difference between the volume of volumetric flask (50.00 ml) and the spent volume of 95% (v/v) ethanol [12].
Adsorption curves for Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+
To elucidate the accumulation mechanisms of Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ in sediments, adsorption curves were built for these cations. For this purpose, sediments were dried and sieved as described in the previous section. Afterwards, adsorption curves were obtained by following a procedure developed in our laboratories, in which 50 mg of sediments were stirred at 200 rpm for 60 minutes with 25.00 ml of different solutions containing Cd2+, Cu2+, Ni2+, Pb2+, or Zn2+ (3 to 10 mgL-1) at pH 7 and 25°C. These solutions were prepared with water of the São Francisco River, which was collected at point 1 of Figure 3 and was previously monitored for total concentrations of these metals. For each concentration used to plot these curves, one analytical blank and three replicates were analyzed. The remaining Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ concentrations in the supernatants were quantified by FAAS. Subsequently, all data that originated adsorption curves were linearized by using the mathematical model of Langmuir, which allows estimation of the following thermodynamic parameters for each one of the mentioned cations: the maximum adsorptive capacity of the sediments, Langmuir constant related to adsorption energy, and separation factor, which predicts the adsorption reversibility.
The analytical quality of all spectrometric quantifications of metals (by FAAS or ICP OES) was checked and confirmed by analyses of standard solutions with traceability for cadmium, chromium, copper, lead, manganese, nickel, and zinc.
Periodic analyses of metals in sediments and water
Table 2 lists the bioavailable concentrations of metals after extraction with 0.1 molL-1 HCl, which is an extractor that simulates the capacity of benthic organisms to assimilate nutrients/pollutants from sediments [9]. This extractor can release adsorbed metals from aluminosilicates and silicates, as well as dissolve alkaline and amphoteric oxides, thus releasing metals that were occluded into or adsorbed onto these oxides.
| Samplings | Sampling points | Cd | Cr | Cu | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| First | 1 | <0.5* | 121.4 ± 7.6 | 3.8 ± 0.2 | 28.6 ± 1.1 | <2.5* | <5.0* | 9.3 ± 0.06 |
| 2 | <0.5 | 377.0 ± 8.9 | <0.5* | 26.3 ± 0.1 | 5.9 ± 0.2 | <5.0 | 13.9 ± 0.07 | |
| 3 | <0.5 | 408.7 ± 9.2 | <0.5 | 20.5 ± 0.7 | 5.7 ± 0.1 | <5.0 | 9.2 ± 0.2 | |
| 4 | <0.5 | 324.4 ± 8.1 | <0.5 | 20.5 ± 0.3 | <2.5 | <5.0 | 4.6 ± 0.09 | |
| 5 | <0.5 | 505.7 ± 19.4 | 7.8 ± 0.2 | 26.3 ± 0.1 | <2.5 | <5.0 | 9.2 ± 0.3 | |
| 6 | <0.5 | 444.3 ± 26.7 | 9.6 ± 0.1 | 37.7 ± 0.5 | <2.5 | <5.0 | 13.2 ± 0.2 | |
| Second | 1 | <0.5 | 86.6 ± 2.4 | 3.1 ± 0.2 | 16.8 ± 1.5 | <2.5 | <5.0 | 7.2 ± 0.3 |
| 2 | <0.5 | 274.4 ± 15.2 | 3.3 ± 0.1 | 87.8 ± 1.1 | <2.5 | 13.2 ± 0.8 | <0.1* | |
| 3 | <0.5 | 501.2 ± 46.4 | 2.8 ± 0.1 | 166.2 ± 1.6 | 7.1 ± 0.6 | <5.0 | 9.4 ± 0.6 | |
| 4 | <0.5 | 456.0 ± 37.4 | 3.3 ± 0.2 | 19.0 ± 0.2 | <2.5 | <5.0 | 5.7 ± 0.2 | |
| 5 | <0.5 | 328.9 ± 11.7 | 1.6 ± 0.2 | 30.8 ± 1.8 | <2.5 | <5.0 | 7.9 ± 0.1 | |
| 6 | <0.5 | 497.7 ± 46.8 | 2.9 ± 0.1 | 171.8 ± 1.8 | <2.5 | <5.0 | 6.1 ± 0.06 | |
| Third | 1 | <0.5 | 174.9 ± 13.6 | <0,5 | 38.9 ± 1.4 | <2.5 | <5.0 | 28.8 ± 0.4 |
| 2 | <0.5 | 404.2 ± 25.5 | <0,5 | 221.9 ± 7.3 | <2.5 | <5.0 | 32.3 ± 0.6 | |
| 3 | <0.5 | 602.7 ± 32.6 | <0,5 | 219.9 ± 2.3 | 4.8 ± 0.4 | <5.0 | 9.4 ± 0.6 | |
| 4 | <0.5 | 517.6 ± 24.8 | <0,5 | 30.9 ± 0.6 | <2.5 | <5.0 | <0.5 | |
| 5 | <0.5 | 577.6 ± 48.9 | <0,5 | 142.2 ± 2.4 | <2.5 | <5.0 | 10.9 ± 0.9 | |
| 6 | <0.5 | 416.6 ± 21.4 | 1.9 ± 0.1 | 129.8 ± 1.4 | <2.5 | <5.0 | 7.7 ± 0.3 | |
| Fourth | 1 | <0.5* | 111.4 ± 5.8 | <0,5* | 18.3 ± 0.9 | 28.6 ± 1.1 | <5.0* | <0.5 |
| 2 | <0.5 | 208.7 ± 21.4 | 2.0 ± 0.1 | 165.9 ± 7.5 | 26.3 ± 0.1 | <5.0 | 2.0 ± 0.1 | |
| 3 | <0.5 | 302.9 ± 24.7 | 1.7 ± 0.01 | 230.0 ± 2.6 | 20.5 ± 0.7 | <5.0 | 1.7 ± 0.01 | |
| 4 | <0.5 | 517.9 ± 38.4 | <0.5 | 32.9 ± 0.4 | 20.5 ± 0.3 | <5.0 | <0.5 | |
| 5 | <0.5 | 274.1 ± 11.3 | <0.5 | 111.1 ± 0.7 | 26.3 ± 0.08 | <5.0 | <0.5 | |
| 6 | <0.5 | 413.7 ± 10.7 | <0.5 | 75.4 ± 0.5 | 37.7 ± 0.5 | <5.0 | <0.5 | |
| Fifth | 1 | <0.5 | 398.5 ± 25.3 | <0.5* | 67.2 ± 1.1 | <2.5* | <5.0* | 7.2 ± 0.5 |
| 2 | <0.5 | 778.4 ± 37.4 | <0.5 | 127.3 ± 8.5 | <2.5 | <5.0 | 14.3 ± 0.9 | |
| 3 | <0.5 | 587.3 ± 46.6 | 2.2 ± 0.1 | 230.9 ± 6.2 | <2.5 | <5.0 | 10.1 ± 0.8 | |
| 4 | <0.5 | 422.2 ± 26.4 | 5.8 ± 0.9 | 198.2 ± 9.1 | <2.5 | <5.0 | 38.0 ± 2.0 | |
| 5 | <0.5 | 314.7 ± 9.9 | 9.3 ± 0.8 | 80.2 ± 6.2 | <2.5 | <5.0 | 23.3 ± 1.8 | |
| 6 | <0.5 | 187.6 ± 17.3 | 6.2 ± 0.9 | 274.3 ± 6.2 | <2.5 | <5.0 | 29.4 ± 2.1 | |
| Sixth | 1 | <0.5 | 329.4 ± 28.8 | <0.5* | 43.7 ± 2.4 | <2.5* | <5.0* | 11.2 ± 0.7 |
| 2 | <0.5 | 429.3 ± 17.7 | <0.5 | 99.4 ± 4.2 | <2.5 | <5.0 | 19.7 ± 0.6 | |
| 3 | <0.5 | 217.1 ± 7.1 | <0.5 | 335.7 ± 5.2 | <2.5 | <5.0 | 7.7 ± 0.4 | |
| 4 | <0.5 | 660.4 ± 34.4 | <0.5 | 176.1 ± 3.0 | <2.5 | <5.0 | 23.1 ± 1.1 | |
| 5 | <0.5 | 523.6 ± 24.0 | <0.5 | 128.1 ± 5.1 | <2.5 | <5.0 | 43.4 ± 2.5 | |
| 6 | <0.5 | 404.5 ± 31.9 | <0,5 | 204.2 ± 4.8 | <2.5 | < 5.0 | 37.9 ± 1.7 |
Table 2: Bioavailable contents of metals (mg kg-1) in sediments from points indicated in Figure 2 (N=3).
Bioavailable Cr concentrations were much higher than 90 mgkg-1, which is the guiding value for fluvial sediments [13]. At points 5 and 6 (Figure 2), the high concentrations of Cr in sediments can be attributed to the continuous discharges of untreated leather tanning effluents, whose composition is rich in Cr3+. At both sampling points, which are close to this type of industry, Cr was precipitated as Cr(OH)3 due to the water pH, which had an average of 7.2 (6.9 to 8.3) for 36 samples. At pH 7.2, precipitations of Cr(OH)3 are expected at [Cr3+] concentrations as small as 1.2 × 10-7 molL-1. Because of the high tendency of Cr to form insoluble Cr(OH)3, total concentration of this element in water was smaller than its analytical detection limit (0.05 mg L-1).
Another source of Cr in the sediments at points 5 and 6 is the erosion of soils naturally enriched with chromite (Cr2O3) [14], whose presence also explains the concentrations of Cr at points 1, 2, 3 and 4 which are located upstream of the tanneries. For comparison, fluvial sediments of another Brazilian area heavily polluted by tanneries had total concentrations of Cr as elevated as 2,878 ± 78 mgkg-1 [15]. Sediments from the Dunajec River (Poland) also showed alarming concentrations of chromium, which exceeded 1,000 mg kg-1 at some sampling points close to tanneries [16]. In turn, average chromium concentrations of 36.67 and 74 mg kg-1 were found in sediments of nonpolluted (by chromium) Iranian rivers [17,18].
The compounds Cr(OH)3 and Cr2O3 have trivalent Cr whose prolonged ingestion can cause stomach cancer in humans [3]. This excessive ingestion of trivalent Cr can come from the eating of benthic organisms (mainly fishes) that live directly in contact with the contaminated sediments. The alarming bioavailable concentrations of trivalent Cr in sediments between Juazeiro, BA and Petrolina, PE can be readily disseminated through the food chain, eventually reaching humans. It is important to note that local cuisine is based on the consumption of native fishes of the São Francisco. Moreover, for many needy families of both cities, the ingesting of São Francisco fishes is the main nutritional option. Therefore, there is an imminent risk related to public health.
Total concentrations of Mn were not detectable (<0.03 mgL-1) in water samples. On the other hand, bioavailable Mn concentrations in sediments were also much higher than those found for Cd, Cu, Pb, Ni, and Zn, although the highest Mn concentration (230.9 ± 6.2 mgkg-1) was lower than 300 mgkg-1, which is considered an environmental limit for sediments [19].
Mn is commonly found in sediments as insoluble pyrolusite (MnO2), which is formed from modifications of other minerals such as rhodochrosite (MnCO3). Once in the sediments, pyrolusite can be reduced to Mn2+ by a reaction that is represented in Equation 1. In this case, electrons would come from organic matter or reduced inorganic compounds.
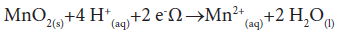 (1)
(1)
However, at the average pH of the São Francisco water (7.2), the transfer of electrons to pyrolusite is limited by the small concentrations of protons. Moreover, this limited concentration of protons also ensures that Mn2+ ions directly released into water column, from some natural or anthropogenic source, are converted to MnO2. The chemical conditions of this water favor the accumulation/maintenance of Mn as MnO2 in sediments, thus explaining the undetectable concentrations of this element in water.
Mn dioxide (MnO2) has considerable toxicity to the human gastrointestinal system [4] if high doses of this compound are ingested. Similarly to the insoluble Cr compounds, the risks of excessive assimilation of MnO2 by humans can be readily realized by the consumption of contaminated benthic organisms.
As discussed above, water pH of the evaluated fluvial section favors accumulation and/or maintenance of insoluble compounds of Cr and Mn in sediments. Nevertheless, the same reasoning cannot be extended to Cd, Cu, Ni, Pb, and Zn, whose hydroxides require much higher water pH to be formed. At the average water pH (7.2) of the São Francisco, precipitations of Cd(OH)2, Cu(OH)2, Ni(OH)2, Pb(OH)2, and Zn(OH)2 would occur at high Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ concentrations [20], which are highly unlikely to be found in a river as voluminous as the São Francisco, even considering the worst cases of aquatic pollution. Thus, if Cd, Cu, Pb, Ni, and Zn were introduced into the São Francisco water, they would be stabilized as Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+. Between Juazeiro, BA, and Petrolina, PE, possible sources of these cations include untreated industrial and/or agricultural wastes, as well as natural weathering of minerals and rocks.
Once in water, Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ can be electrostatically adsorbed on sediment particles, whose superficial chemical groups (hydroxyl, carboxyl, and silanol, among others) are deprotonated at the São Francisco’s water pH, thus generating negative charges. In fluvial waters, dissolved organic matter can form stable complexes with Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ and if these complexes have positive charges or are neutral, they could also be adsorbed on sediment particles. Nevertheless, all water samples had chemical oxygen demand lower than analytical detection limits (0.5 mgL-1), indicating small concentrations of dissolved organic matter, independent of its biodegradable or recalcitrant nature. The small values of chemical oxygen demand minimize the occurrence of complexation reactions involving Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+
All water samples had total concentrations of Cd, Cu, Pb, Ni, and Zn smaller than the analytical detection limits (in mgL-1): 0.001 (Cd), 0.02 (Cu), 0.03 (Ni), 0.05 (Pb), and 0.03 (Zn). This outcome indicates that, at the sampling points, there were not active emissions sources. of these elements. Furthermore, as listed in Table 2, bioavailable levels of Cd, Cu, Ni, Pb, and Zn in sediments were much smaller than their average concentrations in the upper Earth’s crust (mgkg-1): 0.102 (Cd), 14.3 (Cu), 18.6 (Ni), 20 (Pb), and 71 (Zn) [21]. Together with undetectable concentrations in water, the low concentrations of Cd, Cu, Pb, Ni, and Zn in sediments reinforce the finding of limited distribution and accumulation of these metals in the evaluated fluvial section.
For comparative purposes, maximum bioavailable concentrations of Cd, Cu, Ni, Pb, and Zn in fluvial sediments of an industrialized areas of Brazil were, respectively: 4.5 ± 0.0, 17.5 ± 0.2, 4.4 ± 0.4, 5,281 ± 119, and 5,247 ± 452 mgkg-1 [9] respectively. In turn, sediments of another Brazilian river exhibited the following maximum bioavailable concentrations of cadmium, copper, lead and zinc: 1.3 ± 0.03, 44.6 ± 0.4, 33.4 ± 4.0, and 43.7 ± 0.2 mg kg-1 [22]. In the present work, the bioavailable concentrations of nickel in sediments (fourth sampling) (Table 2) were comparable to those found in Odra River (Poland) [23].
Over a long period of time, low contents of Cd, Cu, Pb, Ni, and Zn in sediments do not necessarily imply low risks of human contamination, since the extension of this contamination depends strongly on adsorption reversibility. Thus, if adsorptions of these metals have small reversibility, they can be progressively accumulated in sediments and disseminated through the food chain, eventually reaching humans. To evaluate the reversibility of Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+ adsorptions on sediments, it was necessary to carry out structural analyses and adsorption tests.
Structural analyses of the sediments
To evaluate the greatest possible fluvial extension, sediments were taken at a greater number of sampling points (Figure 3). However, the analyses revealed very similar results and because of this, the discussions concerning the thermogravimetry, infrared spectrophotometry, X-ray diffractometry, and electron scanning microcopy were performed considering only for the sediments collected at point 1.
The sediment thermogram highlights small contents of volatile or thermally unstable compounds (Figure 4), including organic matter. It is possible to observe that only 3% (approximately 0.3 mg) of the initial sediment mass were lost between 250 and 600°C, which is the temperature range in which organic matter and many carbonates typically decompose.
Diffractogram peaks (Figure 5) revealed that quartz is responsible for the most intense peak, while kaolinite, calcite, valerite, hemathite, and illite created the other peaks. This prevalence of quartz indicates sediments with a homogeneous distribution of superficial chemical groups able to adsorb dissolved ions such as Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+. In Table 3, specific mineral structures are associated with their respective 2θ values (Figure 3).
| Mineral | Chemical formula | 2θ value from X-ray diffractogram |
|---|---|---|
| Quartz | SiO2 | 31.062 |
| Hemathite | Fe2O3 | 24.279 e 64.705 |
| Calcite | CaCO3 | 46.192 e 49.728 |
| Valerite | μ-CaCO3 | 52.346 e 58.976 |
| Illite | [K0,8(Al1,8Mg0,2)(Si3,4Al0,6)O10(OH)2 | 10.339 e 20.692 |
| Kaolinite | [Al2Si2O5(OH)4] | 42.724 |
Table 3: Major mineral composition of sediments collected at points of Figure 3.
The infrared spectrum (Figure 6) revealed an intense peak between 1,100 and 1,000 cm-1, which is attributed to the stretch of Si-O-Si bonds of minerals as quartz. No peak associated to common organic functional groups was identified in Figure 6, thus corroborating the small amounts of organic matter previously highlighted by the thermogram (Figure 4). Other Brazilian fluvial sediments with total organic matter of up to 7.8 ± 0.4% (m/m) originated a very distinct infrared spectrum, which exhibited characteristic peaks associated to hydroxyls and carboxyls [22].
The analyses of sediments by electron scanning microscopy revealed particles with heterogeneity in sizes and shapes (Figure 7a and Figure 7b).
Table 4 lists the data concerning the sediment granulometry and density. All sediments had high percentages of sand, which can be explained by erosion of sandy marginal soils [24]. As can be seen in an aerial photo taken between Juazeiro, BA and Petrolina, PE, the fluvial bed was quite silty during the samplings (Figure 8). Due to the accelerated mineral weathering rates, areas with accentuated temperatures tend to have soils enriched with minerals with high chemical resistance (quartz). This is also the case of Cerrado (another Brazilian region subjected to hot weather), where many soils had quartz prevalence in their mineralogical composition [25].
| Sampling point | Sand (%, m/m) |
Silt (%, m/m) |
Clay (%, m/m) |
Density (g cm-3) |
|---|---|---|---|---|
| 1 | 88.3 | 9.4 | 2.3 | 2.66 |
| 2 | 91.7 | 7.6 | 0.7 | 2.74 |
| 3 | 98.7 | 0.5 | 0.8 | 2.74 |
| 4 | 93.8 | 5.2 | 1 | 2.71 |
| 5 | 98.8 | 0.4 | 0.8 | 2.78 |
| 6 | 98.2 | 1.8 | 0 | 2.74 |
| 7 | 98.2 | 1.7 | 0.2 | 2.7 |
| 8 | 98.9 | 0.9 | 0.2 | 2.66 |
| 9 | 98.9 | 0.8 | 0.3 | 2.74 |
Table 4: Granulometric fractioning and density of sediments from points indicated in Figure 3.
A maximum difference of 2.9% was observed between the smallest and the highest density (average value of 2.72 g cm-3), thus revealing great uniformity in the sediment mineral composition. Pure quartz has a density (2.65 g cm-3) close to 2.72 g cm-3, reinforcing the wide distribution of this mineral in the evaluated sediments.
This prevalence of quartz in all sediments suggests great similarity concerning the mechanisms of Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+ adsorptions and values of maximum adsorptive capacities. Moreover, this consistency in the mineralogical composition points to similarities in the adsorption reversibilities for these cations. As discussed below, all these expectations were confirmed.
Adsorption curves for Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+
As previously stated, structural analyses showed the existence of very similar sediments along the points indicated in Figure 3. For this reason, adsorption curves were built with only sediments from point 1. Amounts of Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ that were adsorbed on a fixed sediment mass (qe, mgg-1) and cation equilibrium concentrations in the supernatants (Ce, mgL-1) are listed in Table 5, while the correspondent adsorption curves are indicated in Figures 9a-9e.
| Metallic cation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd2+ | Cu2+ | Ni2+ | Pb2+ | Zn2+ | ||||||||||
| C0 | Ce | Qe | C0 | Ce | Qe | C0 | Ce | Qe | C0 | Ce | Qe | C0 | Ce | Qe |
| 3 | 0.6 | 1.2 | 3 | 0.9 | 1.1 | 3 | 0.9 | 1.1 | 3 | 0.4 | 1.3 | 3 | 1.4 | 0.8 |
| 4 | 1.4 | 1.3 | 4 | 1.7 | 1.2 | 4 | 1.5 | 1.3 | 4 | 1.1 | 1.5 | 4 | 2.3 | 0.9 |
| 5 | 2.3 | 1.4 | 5 | 2.4 | 1.3 | 5 | 2.3 | 1.4 | 5 | 2 | 1.5 | 5 | 2.9 | 1.1 |
| 7 | 4.5 | 1.3 | 7 | 4.8 | 1.1 | 7 | 4.5 | 1.3 | 7 | 4.5 | 1.3 | 7 | 5.4 | 0.8 |
| 8 | 5.3 | 1.4 | 8 | 6.2 | 0.9 | 8 | 6 | 1 | 8 | 5.6 | 1.2 | 8 | 6.2 | 0.9 |
| 10 | 7.2 | 1.4 | 10 | 7.2 | 1.4 | 10 | 6.9 | 1.6 | 10 | 6.2 | 1.9 | 10 | 7.7 | 1.2 |
Table 5: Values of C0 (Initial metallic cation concentration, mg L-1), Ce (Equilibrium metallic cation concentration, mg L-1) and Qe (Adsorbed amount of metallic cation in the sediments, mg g-1) for Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+ adsorption curves.
The Langmuir mathematical model uses the Equation 2 to achieve a linear relationship among the values of qe and Ce. Thus, the closer to 1 the correspondent linear correlation coefficient (R) is, the more the Langmuir mathematical model will fit the treated data derived from an adsorption curve.
In Equation 2, besides the parameters qe and Ce that were already defined, Q0 (mgg-1) is the maximum Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ amounts that can be adsorbed in a fixed sediment mass, and b (Lmg-1) is the constant of Langmuir related to the adsorption energy [26].
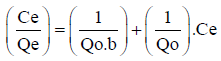 (2)
(2)
It is possible to calculate Qo as the inverse of the angular coefficient (1/Qo) of Equation 2, while the constant of Langmuir (b) can be calculated with the values of Qo and linear coefficient (1/Qob) of this equation.
After applying Equation 2 to the values of qe and Ce (Table 5), line equations and their respective linear correlation coefficients (R) were calculated (Table 6). Table 6 also lists the thermodynamic parameters Qo and b.
| Metallic cation | Line equation | Linear correlation coefficient (R) | Maximum adsorptive capacity Qo (mgg-1) |
Constant of Langmuir b (mgL-1) |
|---|---|---|---|---|
| Cd2+ | Y=0.71X+0.09 | 0.99 | 1.41 | 7.88 |
| Cu2+ | Y=0.88X+0.02 | 0.94 | 1.14 | 43.86 |
| Ni2+ | Y=0.76X+0.09 | 0.93 | 1.31 | 8.48 |
| Pb2+ | Y=0.67X+0.09 | 0.94 | 1.49 | 7.46 |
| Zn2+ | Y=0,91X+0.57 | 0.93 | 1.1 | 1.59 |
Table 6: Data obtained after adsorption curves linearization.
The linear correlation coefficients (R) were ≥ 0.93, thus showing good adjustments of the Langmuir mathematical model to the data that originated the adsorption curves (Figures 9a-9e). This adequacy can be attributed to the large mineralogical similarity among the sediments, thus revealing the existence of adsorptive chemical groups with energetic homogeneity, which is one the most important premises of the Langmuir mathematical model [26].
Although interactions of Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+ with sediments can be efficiently described by adsorptive mechanisms, the extensions of these adsorptions were not appreciable, as indicated by the small values of Qo. The evaluated sediments had maximum adsorptive capacities that were comparatively much smaller than those of other natural materials, including argillaceous minerals [27] and humic organic matter [28]. These limited Qo values agree with the predominance of sandy particles in sediments, which have a small superficial area, few adsorptive chemical groups, and low porosity.
Another important parameter derived from the Langmuir model is the RL (equilibrium or separation factor), which is defined by Equation 3 [29], where b is the Langmuir constant and Co is the highest initial equilibrium cation concentration (10 mgL-1) (Table 5).
RL =1/ (1+ b.Co) (3)
Values of RL between 0 and 1 indicate favorable and reversible adsorptions, while RL=0 is associated to irreversible adsorptions [30,31]. Table 7 lists the RL values that were calculated from b and Co listed in Table 5.
| Metallic cation | RL |
|---|---|
| Cd2+ | 0.013 |
| Cu2+ | 0.0023 |
| Ni2+ | 0.012 |
| Pb2+ | 0.013 |
| Zn2+ | 0.059 |
Table 7: Values of separation factor (RL).
Considering the RL values listed in Table 7, Cd, Cu, Ni, Pb and Zn adsorptions can be classified as favorable and reversible. However, the reversibility’s associated with these adsorptions were small, since RL values were closer to zero than one. It is important to note that RL=0 means total irreversibility [30]. The decreasing reversibility order was: Zn2+>Cd2+>Ni2+>Pb2+>Cu2+. Thus, over a long period of time, alarming amounts of cadmium, copper, lead, nickel and zinc tend to be retained on sediment particles, and accumulated in benthic organisms, eventually reaching humans.
By using an integrated approach, this work assessed the distribution, accumulation mechanisms, and bioavailability of Cd, Cr, Cu, Mn, Ni, Pb and Zn in an important fluvial section of the Brazilian semiarid region, whose environmental conditions had not been studied before.
Considering the average river water pH (7.2), Cr and Mn are settled as insoluble compounds, while Cd, Cu, Ni, Pb and Zn are preferentially retained in sediments by adsorptive processes from their respective hydrated cations. No alarming bioavailable concentrations of Cd, Cu, Ni, Pb and Zn were found in the sediments, but considering their small adsorption reversibility’s, it is possible to expect potential risks related to contamination of benthic organisms. Over long periods of time, this scenario could cause problems concerning human contamination and public health. On the other hand, Cr and Mn, whose bioavailable concentrations in sediments had alarming values, can be readily accumulated in benthic organisms and disseminated through the food chain.
We would like to thank the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), the Research Support Foundation of the State of Bahia (FAPESB) and the State University of Bahia (UNEB) for fellowships and financial support (CNPq: 555522/2006-7 and 620041/2006-4 and FAPEB: APP0076/2009).
The authors declare that there is no conflict of interests regarding the publication of this manuscript.