Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2022)Volume 13, Issue 5
The cellulose/Polyvinyl Alcohol (PVA)/Expanded Graphite (EG) 3D porous foam, which has wide application prospects in cost-effective dye removal, was prepared by physical crosslinking and foaming technology. The prepared foam material has an obvious 3D network and porous structure, exhibiting excellent removal efficiency for Methylene Blue (MB) in an aqueous solution. The largest MB adsorption capacity of the foam is 110.81 mg/g. The adsorption process follows the pseudo-2nd order kinetics and the Freundlich isotherm model, indicating that the adsorption process is controlled by active surface sites and the physical adsorption process. Thermodynamic studies have shown that the adsorption process is a spontaneous and exothermic reaction. After five cycles of adsorption experiments, the composite material still exhibited a more than 70% dye removal rate. The results show that cellulose/PVA/EG 3D porous foam is effective, promising, and recyclable adsorbent, which can be used to remove MB from aqueous solutions.
Long-COVID; PASC; IL-8, IFNγ; Immune exhaustion; SARS-CoV-2
Dyes are widely used in the dying process of textiles, papermaking, pharmaceuticals, food, and other fields [1,2]. A large amount of the wastewater containing organics, which are often toxic, stable in aqueous solution, and difficult for natural degradation, are inevitably produced during the process of dyeing [3,4]. Methylene Blue (MB) is a cationic dye widely used for testing of waste water treatment. At the same time, MB has a huge negative impact on the human body, such as meningitis, neuronal cell apoptosis, nausea, and vomiting [5]. Many methods have already been applied in solving wastewater, such as membrane filtration, flocculation, co-precipitation, chemical redox, and biological treatment [6- 9]. However, these methods have some restricting factors in their application, such as high cost, unfavorable recycling, and secondary pollution.
Adsorption is a common method to sequester organic dyes from wastewater [10]. Compared with other methods, the adsorption shows low cost, high efficiency, easy recovery, and high selectivity [11]. Some traditional adsorbents have been widely discussed. Mahdi Hasanzadeh developed an activated carbon/metal- organic framework composite material to obtain extremely high adsorption capacity in the removal of anionic dyes, and the adsorption process performs the Langmuir model, which indicates chemical adsorption [12]. Lamia Dali Youcef studied the performance of a zeolite in adsorbing cationic dyes. Studies have shown that an excellent dye removal rate can be obtained at a lower dye concentration [13]. Zhihui Huang studied modified bentonite to adsorb Rhodamine B and Acid Red 1. It was found that the maximum adsorption capacity of Rhodamine B and Acid Red 1 were 173.5 mg/g and 157.4 mg/g, respectively [14]. Although these traditional adsorbents are popular, they have poor regeneration ability and are toxic to many organisms. Meanwhile, these adsorbents, based on chemical adsorption during the adsorption process, are hardly reused [15]. Based on the requirements of environment-friendly adsorption, biosorbents have received extensive attention. Among them, cellulose and Polyvinyl Alcohol (PVA) are widely used as biosorbents due to outstanding characteristics such as low cost, solubility, good mechanical strength, stability, biocompatibility, and non-toxicity [16]. Expanded Graphite (EG) is a common carbon material. Compared with graphene, carbon nanotube and graphene oxide have many advantages, such as huge porosity, excellent chemical properties, thermal stability and good mechanical stability, which have significant superiority in adsorption [17,18].
As porous materials have been developed rapidly in recent decades, related research is focused on aerogels and hydrogels. 3D network materials with open porous and microporous structures show great potential in wastewater adsorption [19]. Sheng Tang developed an amphiphilic graphene aerogel for the adsorption of dyes and found the best effect on the adsorption of malachite green [20]. Shu Wang prepared a silk fibroin-modified graphene oxide-based aerogel, which showed excellent adsorption capacity in MB [21]. However, some problems of studied porous materials include expensive raw materials, costly manufacturing, and complicated drying process [22,23].
Foam materials also have an open porous structure, convenient preparation process, and extremely low production cost for wider use [24]. The trimethylammonium grafted cellulose foam prepared by Chuting Feng can selectively adsorb anionic dyes and obtain better adsorption performance [25]. Hong Ma developed flexible cellulose foam to obtain high adsorption capacity (116 mg/g) [26].
In this work, we prepared the cellulose/PVA/EG 3D porous foam by developing a simple and feasible strategy for the removal of methylene blue. After simple foaming and solidification, the prepared foam has a stable 3D and open porous structure, which can efficiently and cyclically remove methylene blue and has broad application in dye wastewater treatment.
Materials
Microcrystalline Cellulose (MCC, 98%, 20 μm), PVA (molecular weight 72000), Sodium Dodecyl Sulfate (SDS, 99%), Sodium Hydroxide (NaOH), Polyethylene Glycol 4000 (PEG), MB, and Ethanol were supplied by Sigma Aldrich (ST. Louis, USA). EG was provided by the Technical University of Liberec. All the solutions used distilled water with 18 MΩ cm electrical resistivity.
Preparation of cellulose solution in PEG+NaOH system
The procedure of cellulose solution was carried out according to the method reported by Yan and Cernencu [27,28]. At first, 2 wt% PEG and 9 wt% NaOH were dissolved in 40 ml of distilled water. Then, 2 wt% MCC was added to this solution, continuously stirring for 3 hours. Finally, the suspension was frozen at –20°C for 12 hours and then thawed at room temperature. This step was repeated three times.
Preparation of 3D porous foam material
At first, 10 wt% PVA and 5 wt% EG were added into the suspension with stirring at 600 rpm and 85° C for 3 hours. Then 2 wt% SDS was added with stirring for 30 minutes to emulsify and foam the suspension. At last, the suspension was frozen for solidification at –20°C for 72 hours. After thawing, the porous foam material was obtained (Figure 1). Herein, samples were named according to the composition of the added chemicals, such as PVA+SDS (PS), MCC+PVA (CP), MCC+PVA+SDS (CPS), and MCC+PVA+SDS+EG (CPSG).
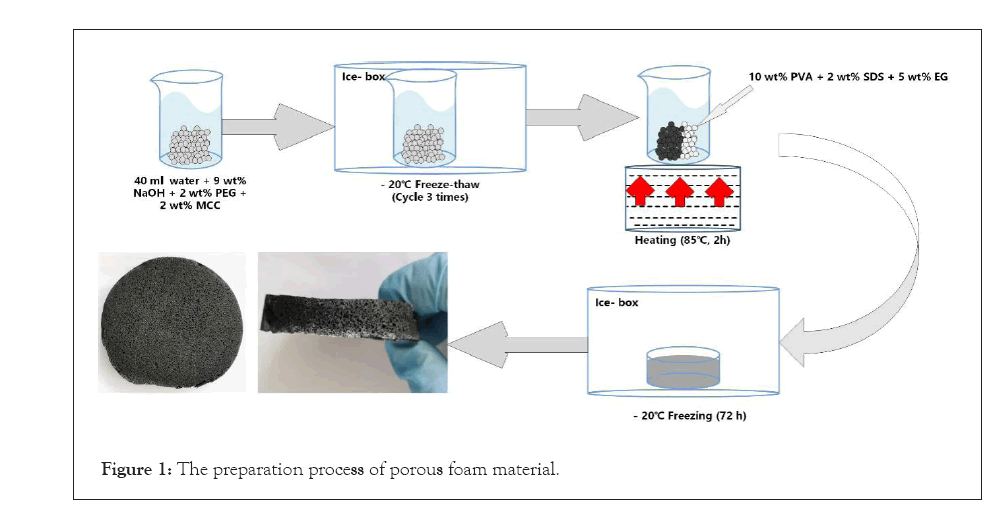
Figure 1: The preparation process of porous foam material.
Morphology test of different composites
For the adsorption experiment, MB was chosen as a model dye to check the adsorption property of porous foam materials. In short, different concentration solutions of MB were prepared by dilution for construction of the calibration curve (Figure 2). Then, we put 0.06 g sample into 30 ml different concentration solution of MB (25 mg/L, 50 mg/L, 100 mg/L, 150 mg/L, 200 mg/L, 250 mg/L). UV-spectrophotometer (7415, Cole-Parmer Ltd.) was utilized to determine the concentration of residual MB dye in the solution after adsorption at 665 nm wavelength. The dye removal efficiency and adsorption capacity were calculated according to equation 1 and 2 [30].
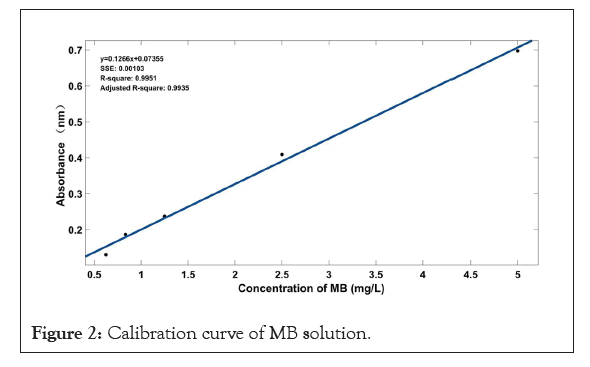
Figure 2: Calibration curve of MB solution.
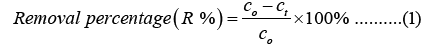
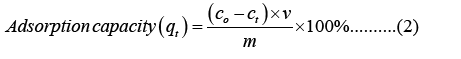
Where c0,ct (mg/L) are the concentrations of MB at zero time and time t, respectively. v is the MB solution volume (L) and m(g) is the mass of the sample.
Recycling test
The sample containing adsorbed MB was placed into absolute ethanol solution for 72 hours at room temperature. Then, the adsorption experiment was repeated after most MB was desorbed from samples.
Surface morphology of different structures
As we can see from Figure 3, it can be clearly observed that CPS and CPSG have a 3D porous structure. In contrast, PS only forms a 2D film, and CP forms a 3D structure without porous structure. At first, as a water-soluble, soft, and long-chain polymer, PVA has widely been applied in the preparation of permeable membrane, aerogel, and hydrogel due to its excellent film-forming and excellent chemical properties [31]. However, even if we added any foaming agent in this research, there was still no porous structure.

Figure 3: Surface morphology. (a: PS; b: CP; c: CPS; d: CPSG; e: front side of CPSG; f: cross-section of CPSG; g: schematic diagram).
This may be because we utilized freezing instead of freeze-drying in this experiment. So, in the subsequent thaw process, its 3D structure collapsed. In addition, bubbles generated by the foaming agent could not form porous structure in the final solidification due to the collapse of the overall structure.
In contrast to CP, CPS and CPSG formed a good 3D structure. The main reason is the addition of cellulose. It is well-known that cellulose consists of a well-organized crystallinity region that contributes to the strength, high stiffness, and amorphous region, which makes fiber more flexible [32,33]. Cellulose is added to the system as reinforcing component filler, forming a uniform network and stable 3D structure in the mixed solution with PVA through hydrogen bonding and supramolecular interaction. Song prepared a cellulose-PVA hydrogel material. They found that after adding cellulose, the mechanical strength of the composite material was significantly enhanced, and the structural stability of the material was improved [34].
Foam molding technology has developed quickly in the past few decades. As an anionic surfactant, SDS has been widely used to foam cellulosic suspensions [35-37]. Lee successfully prepared porous 3D cellulose foam using 3D printing and foaming technology, with good mechanical properties and interesting structure [38]. In this research, cellulose/PVA/EG 3D porous foam was physically cross-linked. Then the PEG and SDS were washed away in the subsequent washing process (Figure 4) and initially utilized SDS as a foaming agent and surfactant to prepare foam material. Compared with CPS and CPSG, SDS is the key to porous forming. Foam is a complex gas/liquid dispersion system. In the effect of high shear force, massive bubbles are generated and accumulated in a trace amount of surfactant solution to form liquid foam slurry [39]. As shown in Figure 3, those irregular bubbles fix the cellulose and EG particles between the gas and liquid phases [40]. In addition, the liquid foam can be prepared by rearranging SDS molecules at the surface of air bubbles entrapped in a suspension [41]. The hydrophobic area of SDS extends out of the bubble surface, and the hydrophilic area combines with water to form a surfactant layer. The negative charge of cellulose and EG caused electrostatic repulsion with SDS. So, they are repelled between the bubbles to form a water layer containing cellulose and EG. Although it has the same charge as cellulose and EG, they still adsorb to hydrophobic sites to form aggregates as SDS is a strong surfactant [42].
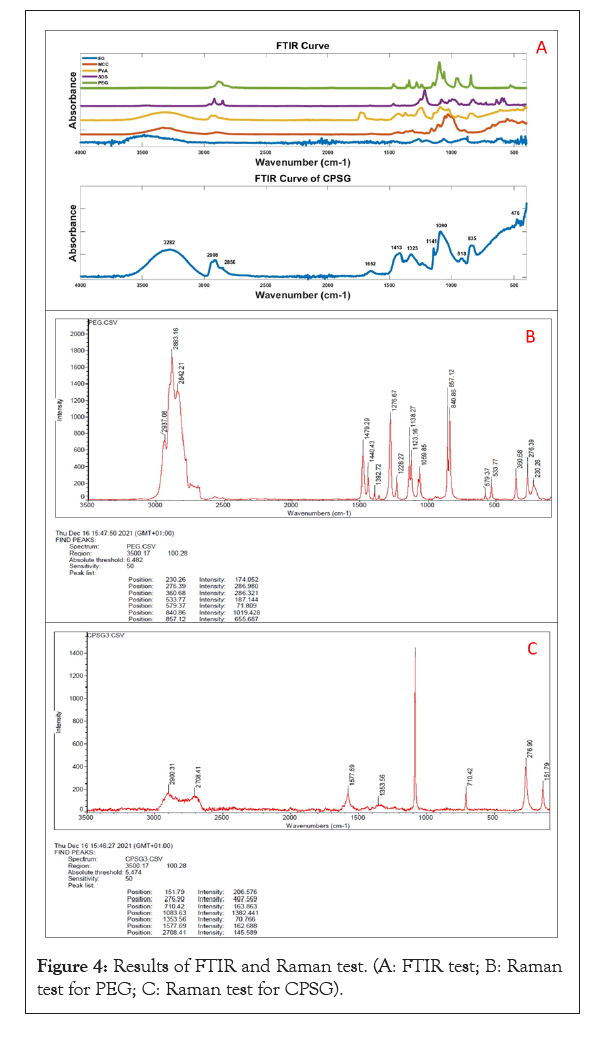
Figure 4: Results of FTIR and Raman test. (A: FTIR test; B: Raman test for PEG; C: Raman test for CPSG).
Influence of different factors on adsorption performance
Adsorption is a surface phenomenon in which the solid surface of the adsorbent is bonded by adsorptive molecules (gas/liquid/solid phase). The whole adsorption process is mainly divided into three steps: (1) Film diffusion is also called external diffusion, in which the adsorbate is transported to the external surface of the adsorbent in the space; (2) Intraparticle Diffusion (IPD), means that the adsorbate diffuses from the external surface of the adsorbent into the pores; (3) Surface reaction, means that the adsorbate is adsorbed on the inner surface of the adsorbent [43,44]. In this case, the mass transfer effect needs to be considered. In the adsorption process, the first two steps are mass transfer, and the last step is the reaction step. The adsorption efficiency is determined by the adsorption resistance. Any step of the adsorption resistance in the above steps will have a huge impact on the total adsorption efficiency [45].
At first, transmission resistance is affected by many factors, including the type and structure of the adsorbent. We investigated the adsorption performance of different samples at relatively lower dye concentrations (25 mg/L). As seen in Figure 5A, CPSG has the highest removal rate of dye (96.21%) and adsorption capacity (12.03 mg/g) compared to CP (16.51%, 2.06 mg/g) and CPS (65.02%, 8.13 mg/g). This is mainly due to the porous structure of CPSG, and MB molecules easily diffuse from the external surface to the internal surface. Moreover, EG provides more active sites and significantly improves the adsorption performance.
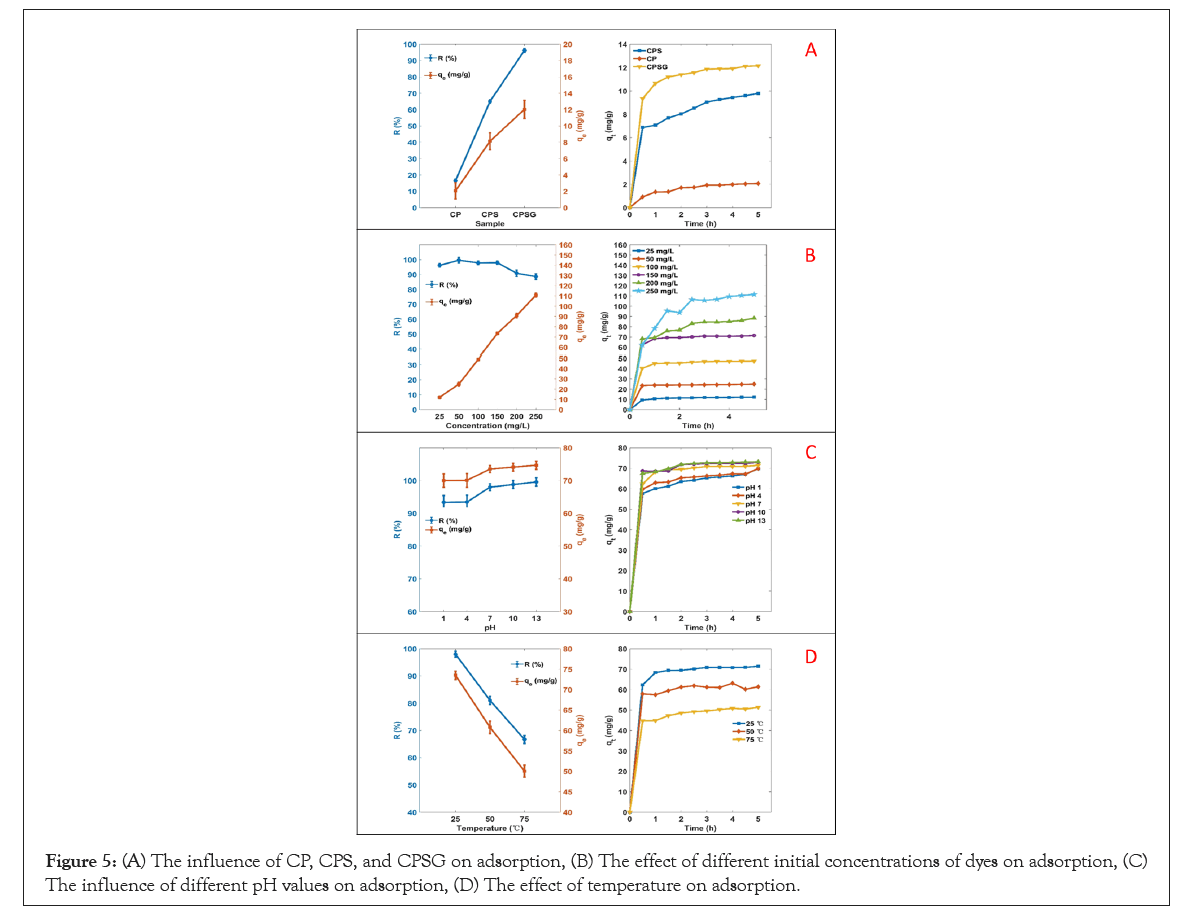
Figure 5: (A) The influence of CP, CPS, and CPSG on adsorption, (B) The effect of different initial concentrations of dyes on adsorption, (C) The influence of different pH values on adsorption, (D) The effect of temperature on adsorption.
In addition, the effect of different initial dye concentrations (25 mg/L–250 mg/L) on the adsorption performance was investigated. It can be seen from Figure 5B that as the initial dye concentration increases, the adsorption capacity increases significantly from 12.03 mg/g to 110.81 mg/g, which is possibly caused by concentration polarization. At higher dye concentration, concentration polarization accelerates the diffusion rate of MB molecules on the surface of the adsorbent, and more dye molecules are adsorbed [46].
The pH value is also one of the important factors influencing adsorption performance. The research results show that the removal rate and adsorption capacity of MB increases with the increase of pH value, which is consistent with the relative researches (Figure 5C) [30,46,47]. At low pH, due to protonation, the positive charge on the surface of the material increases, which causes repulsion between cationic dyes and positively composite, resulting in low adsorption efficiency [48].
Moreover, the influence of different temperatures on the adsorption performance was investigated. It can be seen from Figure 5D that as the temperature increases the dye removal rate and adsorption capacity of the adsorbent decrease significantly, indicating that temperature is also an important influencing factor of the adsorption performance. As the temperature increases, the thermal mobility of dye molecules increases, which may accelerate their transport to the surface of the adsorbent. However, at the same time, it may also accelerate the desorption of dye molecules from the surface of the adsorbent [49].
Adsorption kinetic of adsorbent in MB
There are two possible interactions between adsorbate and adsorbent, namely chemical and physical interaction. Among them, chemical interaction is also called chemical adsorption in that there are chemical or covalent bonds between adsorbate and adsorbent by sharing or transferring electrons. On the other hand, the main force that dominates physical adsorption is van der Waals forces mainly [50].
It is very important to figure out whether an adsorption phenomenon is a chemical or physical adsorption, which can help us understand its adsorption mechanism. Adsorption kinetics reveals the relationship between adsorption efficiency and time and is a good tool to analyze the adsorption rate. This research chooses the three most commonly used kinetic models to study the adsorption rate, as shown in Table 1.
| Kinetic models | Linearized form |
|---|---|
| Pseudo-1st order | 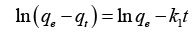 |
| Pseudo-2nd order | 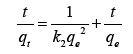 |
| IPD model | 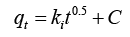 |
Note: Where 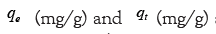 are the adsorption capacity at equilibrium and t time, respectively.
are the adsorption capacity at equilibrium and t time, respectively.  is the time
is the time  and are Pseudo-1st order, Pseudo-2nd order, and IPD rate constant, respectively. is a constant related to the thickness of the boundary layer.
and are Pseudo-1st order, Pseudo-2nd order, and IPD rate constant, respectively. is a constant related to the thickness of the boundary layer.
Table 1: Adsorption kinetic model used in this study.
Batch adsorption experiments were performed with different concentrations of dye solutions at 25°C, and the samples were tested every 30 minutes. The adsorption kinetics curves and parameters are shown in Figure 6 and Table 2. It is obvious that the Pseudo-2nd order is more suitable than the Pseudo-1st order. It reveals that the adsorption process depends on the number of active sites [51].
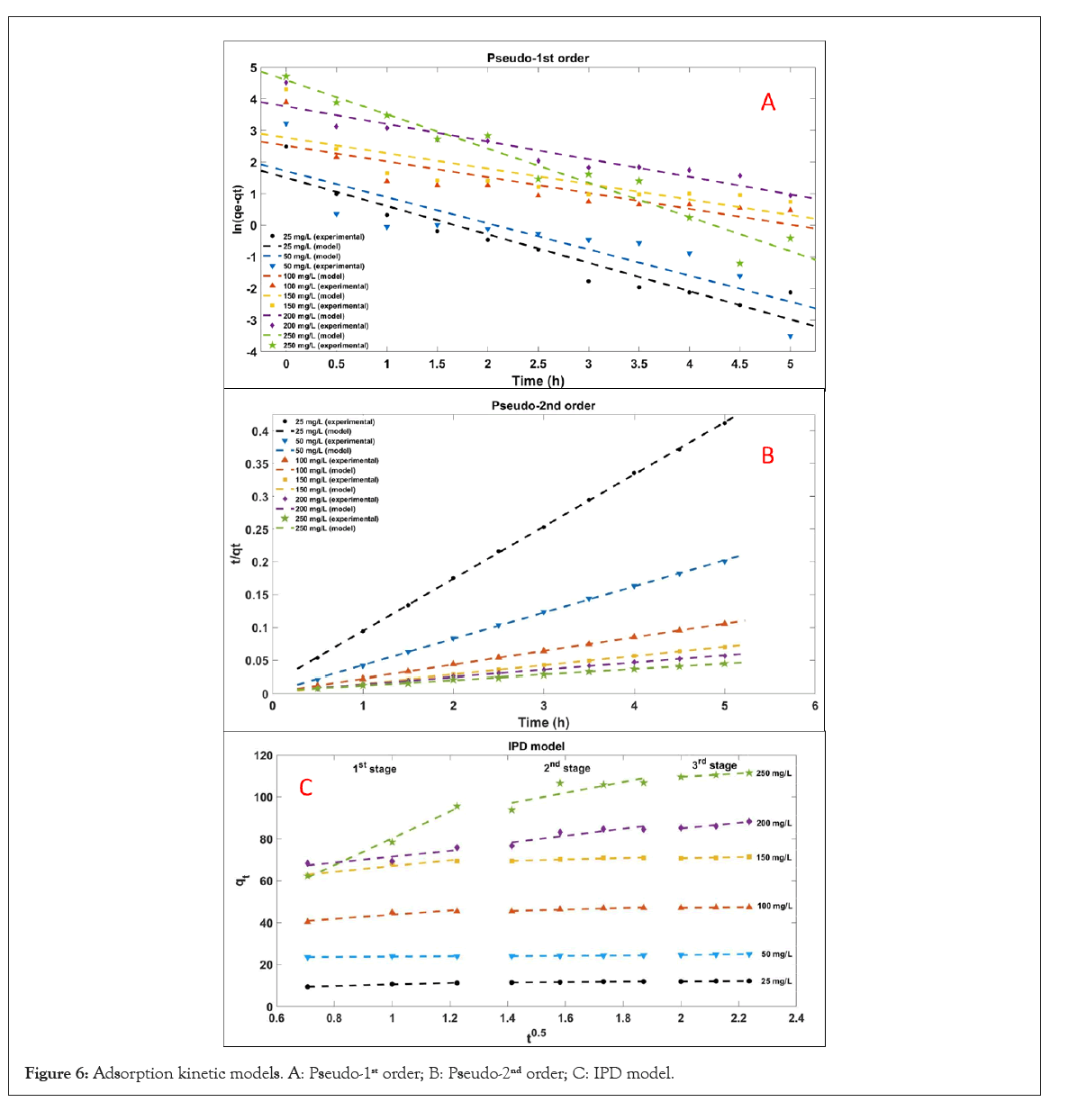
Figure 6: Adsorption kinetic models. A: Pseudo-1st order; B: Pseudo-2nd order; C: IPD model.
| Models | Parameters | 25 mg/L | 50 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | 250 mg/L |
|---|---|---|---|---|---|---|---|
| Pseudo-1st order | qe,exp (mg/L) | 12.03 | 24.91 | 48.94 | 73.49 | 90.9 | 110.81 |
| qe,cal (mg/L) | 4.49 | 5.55 | 12.4 | 15.92 | 42.84 | 98.5 | |
| k1 (min-1) | -0.8963 | -0.8271 | -0.4998 | -0.4887 | -0.5565 | -1.083 | |
| R2 | 0.902 | 0.741 | 0.688 | 0.629 | 0.888 | 0.944 | |
| Pseudo-2nd order | qe,exp (mg/L) | 12.03 | 24.91 | 48.94 | 73.49 | 90.9 | 110.81 |
| qe,cal (mg/L) | 12.56 | 25.06 | 48.19 | 72.2 | 92.17 | 123.03 | |
| k2(min-1) | 0.424 | 0.613 | 0.221 | 0.2014 | 0.037 | 0.0161 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.998 | 0.998 | |
| IPD 1 Stage | ki | 1.86 | 3.64 | 10.07 | 13.97 | 17 | 63.95 |
| C | 20.77 | 6.84 | 33.72 | 53.03 | 54.24 | 16.26 | |
| R2 | 0.998 | 0.976 | 0.872 | 0.911 | 0.768 | 0.992 | |
| IPD 2 Stage | ki | 0.85 | 1.16 | 3.53 | 3.26 | 14.08 | 25.81 |
| C | 22.95 | 9.76 | 40.59 | 64.94 | 57.4 | 60.64 | |
| R2 | 0.715 | 0.939 | 0.915 | 0.912 | 0.797 | 0.649 | |
| IPD 3 Stage | ki | 0.72 | 1.02 | 1.36 | 2.61 | 13.33 | 8.17 |
| C | 23.01 | 9.89 | 44.31 | 65.49 | 58.29 | 93.19 | |
| R2 | 0.986 | 0.882 | 0.984 | 0.878 | 0.941 | 0.999 |
Table 2: Parameters of adsorption kinetic.
However, some research has reported that the kinetic data near equilibrium may have serious deviations if a Pseudo-2nd order kinetic model is applied [52]. Therefore, other models are needed for further study on the adsorption rate.
The IPD model is used to determine the rate-limiting steps of
the entire adsorption process. As shown in Figure 6 and Table 2, 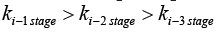 indicates changes in diffusion rate,
revealing that the adsorption process mainly follows three steps:
(1) The first stage is the diffusion of MB molecules from the body
to the external surface of the adsorbent; (2) Then, MB molecules
diffuse through the pores and diffuse into the internal surface;
(3) The last stage is near equilibrium stage. In this stage, the
balance of adsorption and desorption is reached.
indicates changes in diffusion rate,
revealing that the adsorption process mainly follows three steps:
(1) The first stage is the diffusion of MB molecules from the body
to the external surface of the adsorbent; (2) Then, MB molecules
diffuse through the pores and diffuse into the internal surface;
(3) The last stage is near equilibrium stage. In this stage, the
balance of adsorption and desorption is reached.
Adsorption isotherm of adsorbent in MB
The adsorption isotherm is to study the relationship between the concentration of adsorbate in the liquid phase and the adsorbent at a specific temperature at equilibrium. Modeling the equilibrium adsorption data can help us study the adsorption mechanism, the interaction between the adsorbate and the adsorbent, the maximum adsorption capacity, and so on [53]. In this study, several models were selected to analyze the adsorption mechanism (Table 3).
| Isotherm models | Linearized form |
|---|---|
| Langmuir | 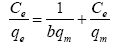 |
| Freundlich | 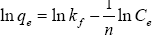 |
| Temkin | 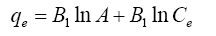 |
Note: Where is the maximum adsorption capacity, 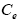 (mg/L) is the equilibrium concentration of dye solution, b is the Langmuir constant, kf and n are Freundlich constants, B1 (Jmol-1) and (L/mg) are the Temkin constant and the equilibrium bond constant.
(mg/L) is the equilibrium concentration of dye solution, b is the Langmuir constant, kf and n are Freundlich constants, B1 (Jmol-1) and (L/mg) are the Temkin constant and the equilibrium bond constant.
Table 3: Adsorption isotherm model used in this study.
It can be seen from Figure 7 and Table 4 that the Freundlich model (R2=0.954, 0.991, 0.979) is more suitable than Langmuir (R2=0.983, 0.863, 0.771) and Temkin model (R2=0.935, 0.871, 0.919) for describing the adsorption of MB molecules on cellulose/ PVA/EG porous materials. It reveals the multilayer adsorption of MB on heterogeneous surfaces. Multilayer adsorption is due to mutual attraction between molecules, and additional molecules are superimposed on the first adsorption layer to form multilayer adsorption. In addition, n represents the adsorption strength of the adsorbent. When n>1, it indicates that the adsorption behavior is favorable [47]. At the same time, porous adsorbent has greater adsorption strength at 25°C as the lowest temperature in the test, indicating that high temperature is unfavorable for the adsorption behavior.
| Parameters | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | kf (L/mg) | n | R2 | B1 (Jmol-1) | A (L/mg) | R2 | |
| 25°C | 112.38 | 0.5161 | 0.983 | 43.1121 | 3.516 | 0.954 | 16.12 | 21.4108 | 0.935 |
| 50°C | 30.3 | 0.0113 | 0.863 | 13.2766 | 2.1404 | 0.991 | 18.06 | 1.5371 | 0.871 |
| 75°C | 70.72 | 0.0048 | 0.771 | 9.8355 | 2.4114 | 0.979 | 13.19 | 1.0879 | 0.919 |
Table 4: Key parameters of adsorption isotherm model.
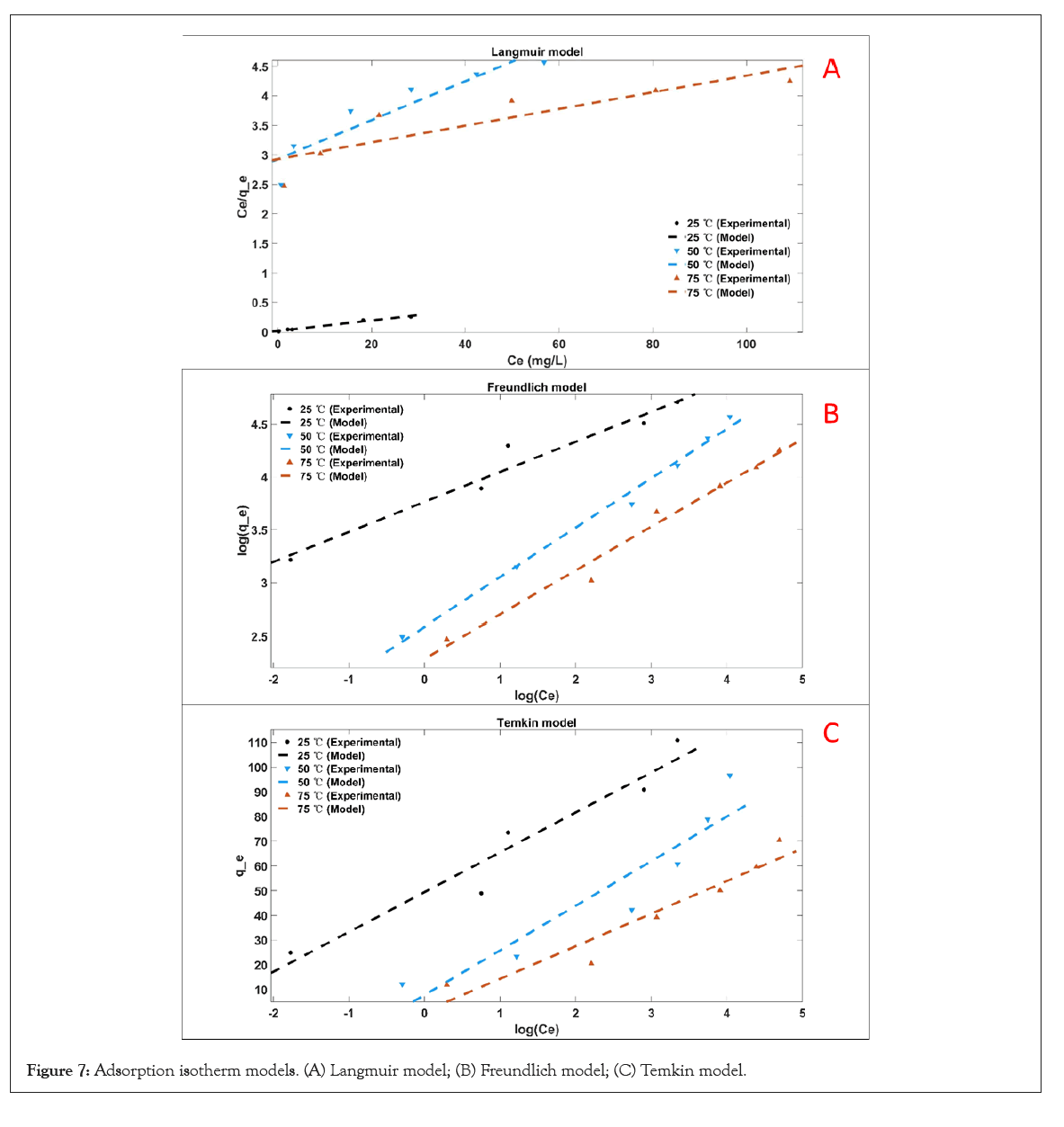
Figure 7: Adsorption isotherm models. (A) Langmuir model; (B) Freundlich model; (C) Temkin model.
Thermodynamic of adsorption
Adsorption thermodynamic is used to survey the trend, degree, and driving force of the adsorption process and plays an important role in explaining the characteristics, laws, and mechanism of adsorption. The changes of parameters of thermodynamic of the adsorption process are generally calculated by equations of Gibbs equation and Vant’ Hoffs equation 3, 4 and 5 [54]:
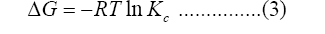
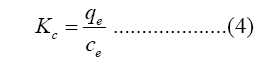
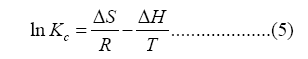
Where ΔG (kJ/mol) is Gibbs free energy, R and T are gas constant (8.314 J/mol/K) and absolute temperature (K), Kc (L/mol) is the equilibrium constant of adsorption, ΔS and ΔH are entropy and enthalpy, respectively.
As shown in Table 5, the free energy ΔG is negative, indicating that the porous material adsorbs MB as a spontaneous process. As the temperature increases, the absolute value of ΔG decreases, indicating that the spontaneous tendency of adsorption decreases, which is not conducive to adsorption? The negative value of the adsorption enthalpy ΔH reveals that the adsorption process is an exothermic reaction. The negative value of the adsorption entropy ΔS indicates that the adsorption reduces the degree of freedom of the adsorbate molecules.
| Parameters | ΔG(kJ/mol) | ΔH(kJ/mol) | ΔS(kJ/mol/K) |
|---|---|---|---|
| 25°C | -7.9121 | -55.7537 | -0.1622 |
| 50°C | -2.0405 | ||
| 75°C | -0.0663 |
Table 5: Parameters of the thermodynamic model.
Reusability of the adsorbent
For industrial applications, the regeneration and recycling of adsorbents are very important. In this study, the adsorbent after adsorption was immersed in an ethanol solution to regenerate it. The experimental result shows that after five cycles, the MB dye removal rate of the composite material drops from 97.98% to 71.93%, possibly for a combination of chemistry and physics adsorption of cellulose/PVA/EG foam. As is known to all, chemical adsorption is irreversible, but physical adsorption is reversible. Therefore, the adsorption process was mainly led by physical adsorption in the subsequent cycle. To sum up, the removal rate of adsorbents after regeneration remains high, which is appropriate for the treatment of MB waste water (Figure 8).
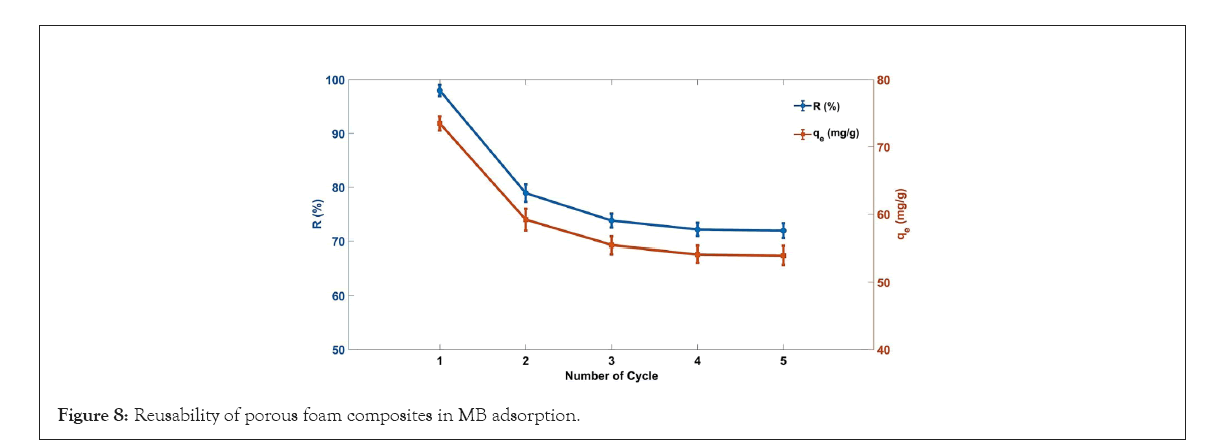
Figure 8: Reusability of porous foam composites in MB adsorption.
Adsorption mechanism of cellulose/PVA/EG foam in MB
The adsorption mechanism of the adsorbate on the adsorbent is affected by many factors, such as the size and structure of the adsorbent. Based on the above experimental results, the adsorption mechanism scheme is listed in Figure 9. According to analyzing adsorption kinetics and isotherm, the adsorption process is mainly physical adsorption affected by the number of active sites and adsorbent structure. So, the possible interactions that determine the effect of MB adsorption are electrostatic adsorption, hydrogen bonding, and van der Waals force.
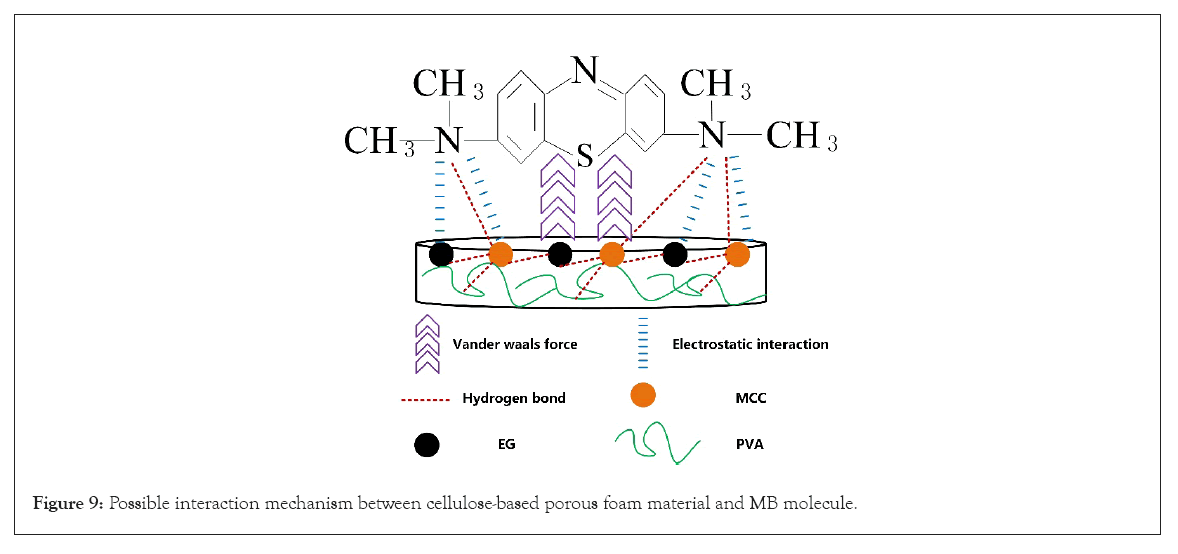
Figure 9: Possible interaction mechanism between cellulose-based porous foam material and MB molecule.
Comparison to other adsorbents of adsorption performance in MB
As shown in Table 6, the adsorbent has significant advantages. The main components of the materials are cellulose, PVA, and EG. These materials are low-cost, renewable, easy to recycle, and degraded. Although the materials developed by Boukhalfa N, and Eltaweil AS have high adsorption capacity [55-56], the cost of their adsorption materials, such as carbon nanotubes or graphene oxide, is very high.
| Adsorbent | Concentration of MB | Removal percentage (%) | qe (mg/g) | Reference |
|---|---|---|---|---|
| Cellulose based porous foam composites | 250 mg/L | 90.9 | 110.8 | This research |
| Maghemite/alginate/functionalized multiwalled carbon nanotubes | 230 mg/L | - | 905.5 | [55] |
| CMC/GOCOOH composite microbeads | 250 mg/L | 72.09 | 180.32 | [56] |
| CMC-Alg/GO hydrogel beads | 15 mg/L | 96.2 | 78.5 | [57] |
| Salecan-g-PAI | 500 mg/L | 22.1 | 107.1 | [58] |
| Magnetic chitosan/clay beads | 100 mg/L | - | 82 | [59] |
| Activated carbon/cellulose biocomposite films | 100 mg/L | - | 103.66 | [60] |
Table 6: Adsorption performance of different adsorbents on MB molecules.
In this study, cellulose/PVA/EG 3D porous foam material was prepared by a simple foaming technique using physical crosslinking, and it was evaluated as a “green” and efficient adsorption material. Results show that cellulose strongly supports the 3D network structure of the material, and SDS effectively promotes the formation of the porous structure. The addition of EG significantly improves the removal efficiency of methylene blue. In the adsorption experiment, the dye concentration, pH, and temperature significantly affect the adsorption result of the material. The adsorption capacity is directly proportional to dye concentration and pH, while the adsorption capacity is inversely proportional to temperature. The adsorption process follows the pseudo-2nd order kinetics and Freundlich isotherm model. Thermodynamic studies have shown that the adsorption process is a spontaneous and exothermic reaction. In addition, after five cycles of adsorption, the repeatability study found that the dye removal rate remained high, although the adsorption capacity of the material decreased slightly. Therefore, the prepared cellulose/PVA/EG 3D porous foam material is a stable, environmentally friendly, efficient, and reusable adsorbent for methylene blue removal.
The work was supported by the project ‘Advanced structures for thermal insulation in extreme conditions’ (Reg. No. 21–32510 M) granted by the Czech Science Foundation (GACR) and was supported by the research project of Student Grant Competition of Technical University of Liberec no. 2022-6069 and no. 2020-6031 granted by Ministry of Education Youth and Sports of Czech Republic.
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Citation: Tan X, Peng Q, Subrova T, Saskov J, Wiener J, Venkataraman M, et al (2022) High Efficient Removal of Methylene Blue Using Microcrystalline Cellulose/Polyvinyl Alcohol/Expanded Graphite 3D Porous Foam as a “Green” Adsorbent. J Thermodyn Catal. 13:308.
Received: 22-Sep-2022, Manuscript No. JTC-22-19327; Editor assigned: 26-Sep-2022, Pre QC No. JTC-22-19327 (PQ); Reviewed: 10-Oct-2022, QC No. JTC-22-19327; Revised: 17-Oct-2022, Manuscript No. JTC-22-19327 (R); Published: 24-Oct-2022 , DOI: 10.37532/2157-7544.22.13.308
Copyright: ©2022 Tan X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.