Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2011) Volume 2, Issue 1
The paper presents the recent advances of water-gas shift process using supported nickel catalysts. The effect of different supports, nickel loading, gas hourly space velocity, dopant materials, on the catalyst activity, H2 yield, and H2 selectivity are discussed. Ceria promoted nickel catalyst supported on powder alumina (Ni/CeO2-Al2O3) demonstrated the best performance. The performance of this catalyst was affected by the amount of nickel loading. The addition of small amounts of cobalt or chromium as a dopant material resulted in a considerable increase of the catalyst performance. The prepared catalysts were also compared with a commercial catalyst (Shift Max 120). It was observed that either of the doped or undoped Ni/CeO2-Al2O3 catalysts revealed a much higher performance in term of activity, H2 yield, and H2 selectivity, as compared to a commercial one.
Water gas shift (WGS) reaction is an established industrial technology in which water (H2O) in the form of steam reacts with carbon monoxide (CO) to produce hydrogen (H2) and carbon dionoxide (CO2). The reaction is presented in Equation 1 [1-3].
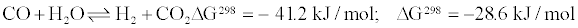 (1)
(1)
Recently, the WGS reaction has attracted new interest due to development on fuel cell technology. Carbon monoxide existed in the synthesis gas produced via steam reforming of hydrocarbons (e.g., natural gas, petroleum, or renewable resources) and gasification of coal or biomass poisons the catalyst used in fuel cell. The benefit of using WGS reaction is that it reduces CO concentration while producing extra H2 which is fuel for hydrogen-fuel-cells.
To produce high purity hydrogen at the highest possible CO conversions, two-stage adiabatic reactors with cooling in between are used: a high temperature shift (HTS) reactor operating at 320– 450°C with a catalyst based on iron oxide structurally promoted with chromium oxide (Fe2O3-Cr2O3), and a low temperature shift (LTS) reactor operating at a temperature range of 200–250°C with copper-zinc oxide supported on alumina (Cu-ZnO/Al2O3) catalyst [4-6]. Typical designs of HTS WGS reactors with Fe2O3-Cr2O3 catalyst reduces CO content from 8–10% to about 3–5% CO, while a LTS reactor with Cu- ZnO/Al2O3 catalysts further decreases the CO level to less than 1%.
It is well known that nickel catalysts are familiar for steam reforming of natural gas. However, Ni also plays an important role for the water gas shift reaction. For example, Willms [7] reported that Ni was a good catalyst to produce hydrogen either through WGS reaction or steam reforming process. Cooper [8] also noted that Ni, which forms a part of the anode composition, facilitates the WGS reaction to take place on the surface of the anode of solid oxide fuel cells. The existence of Ni in CeO2-supported bimetallic Ni-Rh catalyst was reported to help converting CO into CO2 and H2 by WGS reaction [9]. Chu et al. [10] observed that Ni/ceria had higher activity for WGS reaction than Fe/ ceria did. Nickel catalysts, however, seemed to be good for HTS reaction than for the LTS one [10]. Li et al. [11] investigated the use of cerialantana supported catalysts for the WGS reaction at gas hourly space velocity (GHSV) 8000 and 80000 h-1 and temperature ranges of 150 to 550°C. It was found that Ni-Ce(La)Ox catalyst was much superior than the support itself, i.e., Ce(La)Ox. It was also observed that at around 350°C, the activity of Ni-loaded catalyst surpassed the activity of Culoaded one [11]. In the previous works [12,13] we have demonstrated that nickel catalysts showed a good performance for the WGS especially at high temperature (450°C). Our recent report presented that Ni catalysts were not stable at low temperature (250°C), [14] This paper reports the performance of nickel catalysts for high temperature WGS reaction. The experiment was carried out at temperatures 450°C with CO-to-steam (CO/S) molar ratio of 1:3. Effects of different supports, metal loading, GHSV, and dopant materials were investigated. In order to evaluate the prepared catalysts, we also compared with a commercial high temperature WGS catalyst.
The catalysts preparation had been described in our previous work [14]. Nine nickel catalysts with different oxides support are presented in Table 1.
| Catalyst | Composition | BET cm2/g |
|---|---|---|
| Ni/CeO2 | 4% Ni; 96% CeO2 | 80.79 |
| Ni/Al2O3 powder | 4% Ni; 96% Al2O3 | 3.79 |
| Ni/Al2O3 monolith | 4% Ni; 96% Al2O3 | 1.53 |
| Ni/CeZrO4 | 4% Ni; 96% CeO2-ZrO2 | 102.00 |
| Ni/CeYO5 | 4% Ni; 96% CeO2-Y | 100.50 |
| Ni/CeO2-Gd | 4% Ni; 76.8% CeO2; 19.2% Gd | 119.30 |
| Ni/CeO2-Sekar Mirah | 4% Ni; 81.6% CeO2; 14.4% Sm | 425.00 |
| Ni/CeO2-Al2O3 monolith | 4% Ni; 3% CeO2; 93% Al2O3 | 6.44 |
| Ni/CeO2-Al2O3 powder | 4% Ni; 3% CeO2; 93% Al2O3 | 9.01 |
Table 1: The composition of prepared nickel-based catalysts and its BET surface area
| Compound | Fraction |
|---|---|
| Iron (III) oxide | 80-95 |
| Chromium (III) oxide | 5-10 |
| Copper oxide | 1-5 |
| Graphite | 1-5 |
| Chromium (IV) oxide | <5 |
Table 2: Composition (in weight percent) of Shift Max 120
The catalysts were tested using rig and method as described in earlier work [14]. In this work, reaction were conducted at temperature at 450°C with a CO flowrate of 15 ccm, steam flowrate of 0.04 ml/min and catalyst loading of 0.05 g diluted in 1.5 g of fused SiO2 (Sigma Aldrich). A commercial high temperature WGS catalyst, Shift Max 120, was supplied from Süd Chemie. The commercial catalyst had a tablet form and is designed for the high temperature shift WGS reaction working at 450°C. The composition of this commercial catalyst is presented in Table 2.
The catalyst was crushed and sieved to have a particles size range of 20-60 mesh to be analogous to the other tested catalysts. According to the company manual [15], the catalyst must be reduced using syngas at a speed velocity greater than 200 Nm3/h and at a temperature not exceeding 175°C. In this experiment, the catalyst was reduced in situ at 160°C for 7 hours using 15 ccm of gas mixture and 0.15 cm3/min of steam. The composition of gas mixture for reduction step is presented in Table 3. The catalyst was tested at 450°C with a CO flowrate of 75 ccm and a steam flowrate of 0.2 cm3/min.
| Compound | Formula | Fraction |
|---|---|---|
| Acetylene | C2H2 | 2.94 |
| Ethane | C2H6 | 2.85 |
| Ethylene | C2H4 | 2.98 |
| Methyl acetylene | C3H4 | 3.07 |
| Propane | C3H8 | 2.85 |
| Propylene | C3H6 | 3.11 |
| Carbon dioxide | CO2 | 10.30 |
| Methane | CH4 | 10.20 |
| Carbon monoxide | CO | 25.00 |
| Hydrogen | H2 | 29.80 |
| Nitrogen | N2 | balance |
Table 3: Gas composition (in volume percent) used for reduction of Shift Max 120
Catalyst performance is demonstrated by catalyst activity, H2 yield (vol.%), and H2 selectivity. The catalyst activity is presented as CO conversion (XCO) and defined as:
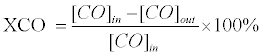 (2)
(2)
Hydrogen selectivity (SH2) is defined as follows:
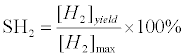 (3)
(3)
in which [H2]max is the maximum H2 yield based on thermodynamic equilibrium at the respected temperature and CO/S ratio.
Effect of support
Figure 1 demonstrated the effect of different supports on the catalyst performance in term of catalyst activity (XCO), H2 yield, and H2 selectivity (SH2). It can be observed that at high temperature all catalysts showed a good performance. Four catalysts including Ni/ CeO2-Al2O3 (powder), Ni/CeO2, Ni/CeYO5, and Ni/CeO2-Gd exhibited very good activity. Except for Ni/CeO2-Gd, the other three catalysts also demonstrated extremely high H2 yield and good stability over 12 h. As can be observed from Figure 2, Ni/CeO2-Gd also took longer time to be stable. Two catalysts, Ni/CeO2-Al2O3 (powder) and Ni/CeO2, had H2 selectivity >70%.
In general, ceria promoted nickel catalyst supported on alumina powder demonstrated the best performance for HTS WGS reaction. At CO/S ratio 1:3, the catalyst had an average activity of 95%, H2 yield of 52% (v/v), and H2 selectivity of 73%. At the same conditions, the equilibrium CO conversion is 94% with H2 yield of 50%. The differences may be resulted from the accuracy of flowrate reading which, in fact, fluctuated from the setting point. Our results, with an acceptable error, suggest that the performance of Ni/CeO2-Al2O3 catalyst was very active for HTS WGS reaction and achieved equilibrium CO conversion even at very little loading (0.05 g).
It can also be observed that catalysts without ceria (Ni/Al2O3, either powder or monolith) had the lowest H2 yield, CO conversion, and H2 selectivity compared to those supported on or promoted with ceria. This observation provides evidence that the presence of ceria is advantageous for WGS catalysts. The beneficial role of ceria for WGS catalyst has been reported, among others, by Hilaire et al. [16], Gorte and Zhao [17], and Swartz et al. [18].
Most catalysts, however, also produced unwanted CH4. The evolution of CH4 formation during WGS reaction is presented as follows:
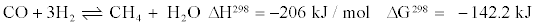 (4)
(4)
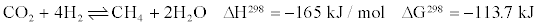 (5)
(5)
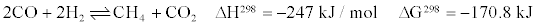 (6)
(6)
Tanaka and Iizuka [19] suggested that after water gas shift reaction, the formation of CH4 occur through the hydrogenation of carbonaceous species formed by the dissociation of CO or CO2 [19]. All of the aforementioned methanation routes require H2. Therefore, the higher the CH4 yield, the lower the H2 yield will be.
Figure 3 shows that monolith alumina supported catalysts (with or without ceria promotion) produced the highest CH4 yield, around 5 vol.% in average. This was another indication of the drawback of using monolith alumina as a support for Ni catalyst.
Effect of GHSV
Gas Hourly Space Velocity (GHSV) is defined as the ratio of the volumetric flow rate of reactants at standard conditions (25°C and 1 atm) to the total catalyst volume [20,21]. If the quantities of catalyst and reactants are in the same units, e.g. for monolith catalyst, GHSV is frequently expressed in h-l (inverse time). For a granule catalyst, GHSV is frequently expressed in ml (gcat.h)-l. A higher GHSV implies a shorter time that the reactants are in contact with catalyst.
Figure 4 shows the effect of GHSV on the performance of 4%Ni/ CeO2-Al2O3. It was revealed that increasing GHSV from 200 to 1000 resulted in the decreasing catalyst performance. A noticeable decrease in CO conversion as the GHSV increase was also observed for the Au/CeO2 catalyst [22] and the Au/TiO2 catalyst [23]. Typical GHSV value for HT or LT WGS reaction is 4000 h-1 [24] but for on-board fuel processing the U.S. Department of Energy targets at least 30,000 h-1 [25,26]. Due to the inverse relationship between GHSV and space time, it is clear that CO conversion will increase with the increasing space time. Our results, as presented by Figure 4 provide evidence for this proposition.
Effect of Ni loading
From the above discussion, it can be concluded that Ni/CeO2-Al2O3 powder is the best catalyst for the WGS reaction at high temperature (450°C). We were interested in investigating the effect of Ni loading on the catalyst performance. For this purpose, we tested the Ni/CeO2- Al2O3 powder catalyst with a different Ni loading ranging from 1 to 8% (w/w). In this test, we also increased the flowrate five times while keeping the other conditions the same. The results are presented in Figure 5.
It can be observed that increasing Ni loading results in a considerable increase of the catalyst performance. Catalyst activity increases from 24% at a Ni loading of 1%; to 54% at a Ni loading of 4%; and to 76% at a Ni loading of 8%. Similarly, H2 yield increases from 15% to 36% and to 44% at Ni loadings of 1%, 2%, and 8% respectively. Hydrogen selectivity also increases from 15% to 55% and to 82% at Ni loadings of 1%, 2%, and 8%, respectively. Again, we observed here that the high H2 yields are followed by higher CH4 production.
Effect of dopant
Figure 6 demonstrates the effect of the addition of slight amounts of a dopant to the Ni/CeO2-Al2O3 powder catalyst. The dopants used here include Co, Cr, Mo, and Ru. These materials were selected because they have been tried as promoters to improve other catalystic reactions. Andreev et al. [22] for example, studied the effect of addition of CoO (5 wt. %) on the activity of Fe–Cr catalysts [27]. Chromium is well known as promoter in commercial iron-based catalysts for high temperature WGS reaction. Meanwhile, the addition of Ru was reported to have a promoting effect on the activity and enhanced the redox effect of the iron oxide catalysts for the WGS reaction [28,29]. Molybdenum and Co are also well known dopants in sulfur-resistant WGS catalysts [30,31].
In our experiment, the loading of the dopant was 1% of the nickel loading and was loaded after the catalyst had been dried. Chromium nitrate nonahydrate (Cr(NO3)3.9H2O), cobalt nitrate hexahydrate (Co(NO3)2.6H2O), ruthenium (III) nitrosyl nitrate (HN4O10Ru) – all from Sigma Aldrich– and ammonium molybdate tetrahydrate ((NH4)6Mo7O24.4H2O) from Fisher Chemicals, were used as precursors for Cr, Co, Ru, and Mo, respectively. The catalysts were tested with reaction conditions of: temperature 450°C, CO flowrate 75 ccm, H2O flowrate 0.2 cm3/min, and catalyst loading 50 mg diluted with 1.5 gram inert silica. The results are presented in Figure 6.
It can be observed that Co- and Cr-doped catalysts substantially improve the catalyst performance in terms of activity, H2 yield, H2 selectivity, and CH4 yield, compared to that of undoped one/s. The undoped, Co-doped, and Cr-doped Ni catalysts had activity values of 48.8, 84.7, and 77.8%, respectively; hydrogen yields of 36.3, 48.9, and 45.8 vol.%, respectively; and hydrogen selectivity values of 60.6, 98.3, and 77.8%, respectively. The addition of Ru dopant resulted in a higher CO conversion (59.7%) than that of the undoped catalyst. However, H2 yield (38.6 vol.%) and H2 selectivity (61.9%) of Ru-doped catalyst were comparable to that of the undoped one. The addition of Mo, on the other hand, resulted in a lower performance than that of the undoped one. This could be attributed to the poisoning effect of Mo on the nickel catalyst. The negative effect of the presence of Mo on other catalysts was also reported by Zhao and Gorte [32], for example, ceria supported palladium [32].
Comparison with commercial catalyst
Figure 7 reveals the performance of the commercial catalyst Shift Max 120 (Süd Chemie). The catalyst is designed for the high temperature shift WGS reaction working at 450°C with composition as presented in Table 2.
It was observed that the commercial catalyst was very stable and was not selective towards CH4 production. However, Ni/CeO2-Al2O3 catalysts, irrespective of being doped or undoped, proved to be much active for the HT WGS reaction compared to the commercial catalyst at identical conditions. The activity of the undoped Ni/CeO2-Al2O3 at 450°C was 60% with a H2 yield of 40 vol.% and SH2 of 60%. Using Cr dopant, the activity of the catalyst was increased to be more than 80% with a H2 yield of 50 vol.% and a H2 selectivity almost 100% (Figure 6). At the same conditions, the activity of the commercial catalyst was 36% with a H2 yield and SH2 of 20 vol.% and 18%, respectively.
Based on the discussion above, it can be concluded that ceriapromoted Ni catalyst supported on alumina powder (Ni/CeO2-Al2O3) demonstrated the best performance for the WGS reaction at high temperature (450°C). The addition of a small amount of Cr or Co as a dopant considerably increased the performance of Ni/CeO2-Al2O3 catalyst. Compared to a commercial catalyst for high temperature shift WGS, both doped and undoped Ni/CeO2-Al2O3 catalysts demonstrated higher activity, H2 yield and H2 selectivity. The importance of this work is that Ni catalyst developed is non-pyrophoric. The other important feature is that there is no need to reduce the catalysts prior to use.