Emergency Medicine: Open Access
Open Access
ISSN: 2165-7548
ISSN: 2165-7548
Research Article - (2015) Volume 5, Issue 4
Keywords: Malaria; HIV; Children; Bobo-Dioulasso; Burkina Faso
Malaria and HIV infection are nowadays two major public health issues in sub-Saharan Africa. They are the leading causes of morbidity and mortality. Indeed they claim more than 4 million lives yearly, mostly in developing countries. Each a minimum of 50 million pregnancies are recorded with women living in malaria-endemic areas, with about half of them in sub-Saharan Africa. In this part of Africa, it is estimated that one million pregnancies each year occur with women suffering from an HIV- malaria co infection [1].
Co-infection is much encountered with children in this part of the world, since vertical transmission is the main route of HIV transmission to children. In fact, according to statistics, a child is subject to at least one fit of malaria each transmission season. Malarial transmission is prevalent in the endemic epidemic mode with an increase in the rainy season (from July to October). With regards to HIV infection, there were 10,000 children infected with HIV, living in Burkina Faso in 2008. Bobo-Dioulasso is among the cities where Burkina Faso where the prevalence of HIV infection is high. In 2007 it was estimated at 3%, while the national prevalence was 2%. The profound impact of these infections on the immune system is generating interest in the scientific community [2]. Indeed, previous studies reported that HIV infection would be responsible for an increased incidence of malaria attacks and an increase in parasitaemia [3-6]. However, this finding is not shared by all; in fact, some authors have been reporting low malaria prevalence (11.4%) with children infected with HIV compared to HIV-negative children (27.6%) [7]. HIV- co infection malaria may also pose diagnostic problems. Indeed, for some authors high parasitaemia would be associated with a high prevalence of “false positive” of immune-enzyme tests, especially with young patients [8,9]. Furthermore, therapeutic issues were raised by some authors [10,11].
Thus, the combination therapies based on artemisinin (CTA) would have potential interactions with Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) and protease inhibitors (PIs) that are metabolized by CYP450 [1]. Also, the co-administration of sulfadoxine-pyrimethamine combination (SP) with cotrimoxazole or nevirapine increases the risk of liver and skin side effects, and with zidovudine, the risk of bone marrow toxicity.
Evolutionary interactions between these two diseases have been reported by other authors. In fact, during the acute phase of malaria the viral load of HIV would raise, increasing the risk of transmission, resulting in a decline in CD4 rate [12,13].
This study analyzed the clinical profile, biological and therapeutic HIV-malaria co-infection with children followed at Day hospital (DH) of University Teaching Hospital SOURO SANOU (UTH SS), Bobo-Dioulasso allowed a better understanding of the interaction between these two major diseases in Burkina Faso.
The study was carried out in the Department of Pediatrics of UTH SS, Bobo-Dioulasso in western Burkina Faso. Two types of studies were carried out from July to December 2013:
1- A Prospective cross-sectional study about children whose HIV status was unknown at admittance;
2- A retrospective cross-sectional study about children infected with HIV and followed in the department.
The population1 consisted of HIV positive patients, followed in the department and who’s clinical and laboratory diagnosis of malaria was made.
Population 2 consisted of patients who consulted the pediatric department for a suggestive clinical picture of malaria confirmed by parasitological examination; those with positive HIV serology were involved. The involvement was made after obtaining the informed and free consent of parents or legal guardians.
For population 1 data were collected from the physical and electronic records of patients. And the population 2, the clinical diagnosis was provided by a clinician, and laboratory confirmation of malaria was made from thick blood gouts after colouring with Giemsa 10% and viewed by optical microscope. Positive cases in Search for Plasmodium (SP) were submitted to a rapid HIV screening (Determine®). A confirmation and typifying test was done when the first test was positive. SD Bioline® was used for this purpose. Sociodemographic, clinical, biological, pharmacological therapy data were collected using a questionnaire.
Newborns were aged from 0 to 29 days and infants aged from 1 to 30 months, the toddlers over 30 months to 5 years, as for late toddlers and teenagers their proportion of respective age was from over 5 years to 10 years and from over10 years to 18 years. We considered severe anemia a hemoglobin rate (Hb) less than 7 g / L; moderate anemia an Hb rate between 7 and 9 g/L and a light anemia, an Hb rate, ranging from beyond 9 g and less than or equal to 11g / L. As for CD4 rate, we have taken into account the WHO recommendations of putting on treatment (2010), to classify our patients as follows:
• Children 0-59 months [0-750 [cell/μl = Immunocompromised, 750 and more = immunocompetents.
• Children aged 60 months and over: [0-350 [cell/μl = Immunocompromised, 350 and more = immunocompetents.
The statistical test used was the Chi square test at 5% significance threshold. For low numbers and in cases where one of the expected values in a cell was less than 5, the Yates' correction was applied.
Epidemiological aspects of HIV- Malaria co infection
In population 1: Involved, were children infected with HIV and followed in the department. The number of visits during the study period was 3313 including 102 cases of malaria, i.e. a co infection prevalence of 3.07%.
In population 2: Involved were Children who were diagnosed with malaria at admittance whose HIV status was determined when hospitalized. Out of 181 cases, there were 6 patients infected with HIV and 4 patients put at risk (patients under 18 months who tested positive to the rapid test: Determine®) i.e., a prevalence of 3.3% in this population.
The overall prevalence of co infection was estimated at 3.09%.
Distribution of coinfection per age
The prevalence was higher in the group of teenagers (43.5%), Figure 1 shows the distribution of co-infection per age.
Monthly dynamics of co infection
Malaria and HIV co-infection was more observed during the malaria transmission season (July-October). It was 12.9% (14 cases) in July, 20.4% (20 cases) in August, 20.4% (22 cases) in September and 27.8% in October i.e., 30 cases. Respective rates of 12% (13 cases) and 8% (9cas) were observed in November and December.
Clinical aspects of co infection
The most common clinical signs in the 2 populations were fever, headache and vomiting. Signs of seriousness were dominated in population 1 by consciousness disorders (3.9%), hemoglobinuria (0.98%), convulsion (0.98%) and jaundice (0.98%), whereas in population 2 we noted dehydration (50%), disorders of consciousness (33.3%), coma (16.7%) and seizures (33.3%).
Distribution of patients accordance with the type of malaria
Severe cases of malaria were 14% versus 86% for simple malaria. All patients diagnosed HIV positive at admittance were suffering from severe malaria, while with children infected with HIV and followed up at the Pediatrics Department, severe malaria concerned only 8.8% of cases of co infection.
Classification of patients in accordance with WHO clinical stage
Figure 2 shows the frequency of different clinical stages of HIV infection in accordance with WHO classification. Patients in stage 1 represented the majority of cases (60.6%). There was a significant difference between clinical stage and evolution. The risk of death was higher with patients at advanced stages.
Biological aspects of co infection
In both populations as shown in Figure 3, there was a higher incidence of mild anemia
In population 1, there was 66.7% of mild anemia; 22.8% of moderate anemia and 10.5% of severe anemia. In Population 2, severe anemia was found in 66.6% of patients with an equal distribution of the other two types of anemia i.e., 16.7%.
Leukocytosis was found with 19 patients versus 7 cases of leukopenia. With 71 patients no abnormalities of the leukocyte rate was observed.
In Population 2 50% of patients had leukocytosis versus 17.6% in the population 1 was present with 16 patients were suffering from thrombocytosis and 9 from thrombocytopenia.
The majority of patients showed no platelet defects (74.2%).
Immunological data
Distribution of patients in accordance with their immunological profile: The frequency of HIV-1 infection was 97.2%. We reported a rate of 29.3% of immunocompromised versus 70.7% of immunocompetent. Before 60 months there was a neat predominance of immunosuppression (71.4%), conversely after 60 months, immunocompetence predominated (75%).
Distribution of patients in accordance with the CD4 rate and type of malaria: Children under 60 months suffered all from uncomplicated malaria. With the 60 months old and more, uncomplicated malaria had an almost equal distribution between immunocompetent and immunocompromised with respective rates of 90% and 88%. However, severe malaria was found with at a higher frequency with immunocompromised (11.8%) versus 9.8% with immunocompetent.
Pharmacotherapeutic support: Antimalarials were prescribed to all patients at respective rates of 86.1% (93/108) and 13.9% (15/108) for amodiaquine/artesunate and quinine, and 48.1% of patients were on antiretroviral therapy (ART).
Table 1 shows the distribution of pharmacotherapeutic groups used in co infection.
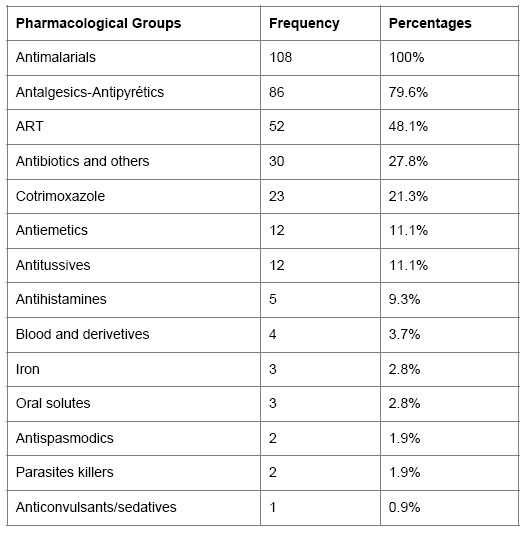
Table 1: Frequency of therapeutic groups’ prescription.
As for ARV treatment, the combination of d4T-3TC-NVP represented the group of ARVs used mostly. The Figure 4 reports the distribution of the various combinations of used ART.
Prevalence of co infection
The overall prevalence was 3.09%. This rate is lower than those reported by Villamor, Tanzania (11.4%) and Whitworth, Uganda (11.8%) [7,14]. However, these two studies estimated the existence of parasitaemia or not with patients infected with HIV and not malaria disease. Thus the prevalence of malaria in their respective studies could be lower because asymptomatic carriage of the parasite in stable endemic countries is common.
The low prevalence of malaria in children infected with HIV is also explained by the compliance with a reinforcement of medical care with these patients, and sensitization of parents and guardians to early medical consultation at the slightest symptom of clinical suspicion of malaria. Kawo shares our results in his study by relating malaria prevalence four times higher with HIV-negative children compared to children infected with HIV [15].
Several hypotheses were put forward to explain this finding including
• Co-trimoxazole prophylaxis, which would significantly reduce the risk of occurrence of malaria in children infected with HIV. In Mali, Thera reported a reduction in the incidence of malaria cases with children under chemo prophylaxis not infected with HIV. Such a finding is shared by several other authors who reported in their work a reduction the incidence of malaria attacks and mortality among HIV-infected patients on cotrimoxazole [10,13,16-18].
• Oxidative stress created by the HIV infection in the erythrocytes that would be unfavorable to the development of Plasmodium [19].
• Antiretroviral therapy would promote the control of malaria infections, firstly, by restoring the antimalarial immune response and on the other, by the effect of some molecules that could have an antimalarial effect in vitro, it is the case of protease inhibitors such as ritonavir and saquinavir which inhibit in vitro the parasite growth at clinically effective concentrations [20,21]. So, ARV treatment by non-nucleoside reverse transcriptase inhibitors would be associated with a decrease in the incidence of malarial attacks without ruling out the involvement of the effects of immune restoration [22]. Thus Mermin brought through his studies an impact of ARV treatment with co infected patients. Indeed, the annual incidence of malaria without intervention was 50.8 episodes per 100 people per year, which was reduced to 9 malaria episodes per 100 people per year with chemoprophylaxis with cotrimoxazole only and 3.5 episodes per 100 people per year with cotrimoxazole and antiretroviral treatment [17].
• The reduction of HIV replication in secondary human macrophages resulting from the presence of Plasmodium haemozoin in endothelial cells of the malaria cured patients or with chronic infection. In in vitro, indeed, this reduction is associated with a restriction on the integration of the viral genome into the chromosome of the target host cells [22].
Distribution of coinfection in accordance with age
In population 1, the prevalence of co-infection was low with those under 24 months. The administration of cotrimoxazole with prophylactic perspective being systematic with HIV-exposed children up to the age of 18 months before showing their non-contamination, could justify this finding.
However in Population 2, children from 0-5 years old accounted for 83% of malaria-stricken population. The high incidence of malaria in this age group is well known in areas of endemic malaria prophylactic and extensive sensitization campaigns are directed towards this target group in Burkina [23].
Temporal distribution of co-infection
The monthly dynamics of malaria transmission in Bobo-Dioulasso may justify the increasing prevalence of malaria from July to October. Indeed in western Burkina Faso, malaria is rampant in an endemic epidemic mode with a high prevalence during the rainy season (July to September) and a peak in early dry season (October) [24].
Clinical profile
Clinical signs: Clinically, the three main reasons for consultation in population 1 were pyrexia (97%), headache (61.8%) and vomiting (37.3%). Within this population signs of severity were pallor (3.9%), disorders of consciousness (3.9%), repeated convulsions (0.98%), hemoglobinuria (0.98%) and jaundice (0.98%). In Population 2, the signs of severity differed from those observed in the population 1 with higher proportions of dehydration (50%), repeated convulsions (50%), disorders of consciousness (33.3%) of pallor (33.3%), coma (16.7%). Regular monitoring of patients infected with HIV and the effectiveness of management protocols for these children explains the low levels of seriousness signs in the population 1. Although these signs are often found in the symptomatology of severe malaria regardless of any HIV infection [25], this difference in distribution of seriousness signs in children infected with HIV and followed, and children whose HIV status was discovered at the moment of suspected signs of malaria should be elucidated [26,27].
WHO clinical stage: The majority of patients were in stage 1 of HIV infection (56%) and death was significantly correlated with WHO clinical stage which was high at the advanced stage of HIV infection. However, the accountability of malaria as a cause of death at this late stage of HIV infection when opportunistic infections are predominant remains difficult to confirm.
Biological profile
Hematology data: In population 1, mild anemia, however, was predominant in the population 2; severe anemia was reported in more than half of this population (66.7%).
The strong correlation severe anemia-HIV infection-severe malaria (whatever the parasite density) in a high transmission area of malaria is also reported by several other authors such as Oura and Otieno and could explain the high prevalence of severe anemia in the population where the severe form of malaria prevailed [19,23].
In both populations, leukocytosis was the most frequent quantitative abnormality of leukocytes. The majority of patients exhibited no quantitative platelet defects (74.2%).
Immunological data: HIV Serotype 1 was predominant and found in 97.2% in our study. The prevalence of this serotype in Africa is known and reported by several authors [14,15]. As it is clear from our series, a high prevalence of immunocompetent individuals (70.7%).
Yet many authors through their studies have reported a drop in CD4 rates during a malaria attack, Mermin and Whitworth, Uganda [14,28]. The unavailability of CD4 rate before malaria with some of our patients (population 2), does not permit the establishment of any link of changes in CD4 levels before, during and after an attack of malaria. But no relationship was observed between the clinical form of malaria and the immunological profile of patients.
Therapeutic profile: The most prescribed antimalarials were the combination amodiaquine - artesunate with a rate of 86.1%. This is explained by the predominance of the simple form of malaria, the diagnosis was made with 86% of our patients, indeed, in accordance with the national guidelines in Burkina Faso, which is part of the strategic plan to fight against malaria (2011-2015), the treatment of uncomplicated malaria uses the combination therapy amodiaquine - artesunate or artemether-lumefantrine by oral route; injectable forms of artesunate, artemether and quinine are reserved for severe forms which concerned only 14% our patients [23].
Co infection does not require changing the therapeutic protocol compared to the treatment of uncomplicated malaria and severe malaria, which is shared by [29], although for some authors a delay was found in the clearance of parasite with patients infected with HIV and treated by means of artemisinin, suggesting a metabolic disorder of antimalarials according to the immunological profile of the host [30-33].
This study showed a low prevalence of HIV-malaria co infection in children. But malaria can be a testing ground for HIV infection. Co infection involved more teenagers than infants with a strong higher frequency during the malaria transmission season. Leukocytosis is the most common laboratory abnormality during this co infection. No relationship was observed between the clinical form of malaria and the immunological profile of patients.
The treatment of malaria during co infection followed national guidelines for the fight against malaria. Signs of severe malaria were less observed in children infected with HIV and followed in Pediatrics.
Therefore, devolution of health structures monitoring children infected with HIV, involving Health Centers and Social Promotion (CSPS), combined with a strengthening of medical monitoring of therapeutic and technical capacities of children infected with HIV malaria is essential.