Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2019)Volume 11, Issue 4
Objective: The goal of the work was to study the socio-demographic and clinical profile of the patients with TB/ HIV co-infection, as well as the treatment effectiveness to understand what shortcomings in the work of TB and HIV services are needed to be corrected.
Materials and methods: A retrospective study was performed among 377 patients with dual co-infection TB/HIV between January 2015 and December 2016. TB diagnoses were based on clinical symptoms, sputum microscopy, and radiological analyses. The patients were diagnosed as HIV seropositive by ELISA and by Western blot.
Results: Out of 377 individuals with co-infection HIV/TB there were 56.8% of patients with newly diagnosed TB. About 30.8% of individuals with newly diagnosed TB did not know they were HIV-positive and attended the hospital for TB treatment. It was revealed that the most predominant TB-form was disseminated pulmonary tuberculosis in the phase of infiltration/decay both among newly diagnosed HIV positive patients and the HIV/ TB patients registered in specialized care centers-50.5% and 49.7%, respectively. The active TB-form (MbT+) accounted for 40.3%. Cavities in the lungs were revealed in 19.9% of patients. The treatment effectiveness cessation of the Mycobacterium tuberculosis allocation was 75.2% in newly diagnosed TB patients and 55.3% in registered patients. Cavity closure had occurred in 54.1% in patients with newly diagnosed TB and 34.2% in registered patients. Only half of the patients (51.1%) constantly took prescribed medications.
Conclusion: The high rate of HIV-infected patients with newly diagnosed TB (56.8%) indicates insufficient effectiveness of programmes for early TB testing. Also, the fact that about 30.8% of individuals with newly diagnosed TB were not aware of their HIV positive status indicates the urgent necessity for optimizing the interaction between TB and HIV services. One should also pay attention to the low patients’ adherence to the treatment as only 47.5% of patients did undergo treatment.
TB/HIV co-infection; TB incidence rate; Patient’ socio-demographic profile; Newly diagnosed TB
One of the leading causes of death worldwide is tuberculosis (TB) associated with HIV. In 2017 about 374 000 people died because of HIV/TB co-infection [1]. According to the World Health Organization (WHO) report out of 10 million new cases of TB registered in 2017, there were 0.9 million new cases of HIV associated tuberculosis. It is a well-known fact that HIV and TB form a deadly combination, each enhancing the other's progress. HIV-positive people have a 21-34 times higher risk of developing tuberculosis than HIV-uninfected people [2,3]. Sonnerberg et al. showed that almost half of HIV-infected individuals after infection with M. tuberculosis develop TB within a year [4]. Moreover, it was confirmed by Getahun et al., Maher et al. and others that HIV infection increases the risk of TB reinfection by 20 times [5-8]. TB/HIV co-infection is characterized by atypical TB clinical manifestations such as a false negative TB sputum smear, normal chest X-ray and a higher rate of extra-pulmonary TB [9]. In turn, the active TB form speeds the progression of HIV infection due to the chronic immune reactivation caused by M. tuberculosis and significantly increases the risk of death [10-15]. Today tuberculosis as a secondary disease is detected in 32%-56% of patients with advanced stages of HIV infection [1].
TB associated with HIV-infection is still a major international and national health problem not only in developing countries but also in economically advanced countries. In the European region, the number of TB/HIV cases amounted considerably since the 2012 year, from 5.5% to 9%, with the majority of cases been registered in Eastern Europe [2].
According to the UNAIDS report, 75% of all new cases of HIV in Europe are registered in Russia. As to TB, Russia occupies the 20th place in the list of countries with high TB burden and it is among the three leading countries in the number of cases of drugresistant TB [16,17]. The percentage of HIV-positive individuals newly diagnosed with TB increased from 3.8% in 2014 up to 8.2% in 2015-2016 and 8.5% in 2017 [18]. In 2017 the incidence of TB in HIV-infected individuals among the population of the Russian Federation amounted up to 1 799.6 per 100,000 population. This is 53.3 times higher than the average TB incidence in Russia among HIV-negative individuals (33.4 per 100,000 populations). According to the experts in Russia every day sixty-two patients die from TB/HIV co-infection [17-19]. However, TB is a curable disease and early detection and treatment of TB in HIV-positive individuals allow considerably diminishing the risk of severe forms of TB and death rate. The goal of the work was to study the socio-demographic and clinical profile of the patients with TB/HIV co-infection, as well as the treatment effectiveness to understand what shortcomings in the work of TB and HIV health facilities it is necessary to pay attention.
A retrospective analysis was performed among HIV-infected patients with TB co-infection who attended the Moscow Tuberculosis Clinic. TB diagnoses were based on clinical symptoms, sputum microscopy, and radiological analyses. The patients were diagnosed as HIV seropositive by ELISA and by Western blot. The CD4+ count was performed by two-color flow cytometry, using phycoerythrin-anti-CD4 (FACSort, Becton Dickinson, USA). Demographic data and clinical characteristics were collected basing on the protocol. Data were collected regarding the probable route of infection (self-reported): sexual either homosexual or heterosexual intercourse or non-sexual; employment status (employed/unemployed); education level. Also, an oral interview was conducted with each of the patients by attending physicians and was registered in the medical record.
A retrospective study was performed among 377 HIV-infected patients with TB co-infection between January 2015 and December 2016. Among the patients’ group, men accounted for 64.5% (Table 1). The median age of the study population was 37.9 (24÷62) years for males and 35.4(22÷72) years for females. The age group 30-39 years prevailed both among men and women (73.3% and 54.5%, respectively), followed by 21% in the age group 40-49 years. The ratio of patients in the age group 50-55 years for women was higher than for men-10.4% versus 2.5%, respectively. The socioeconomic status of patients was rather low. The majority of the patients had secondary and secondary-special level education that is 368 (97.6%). University-level education had only 9 (2.4%) individuals. Seventy-nine individuals (20.9%) had a regular job. Out of those 7 (8.9%) had a university degree and 72 (91.1%) had secondary education. Out of 298 (79.1%) patients who did not work, 86 (28.9%) individuals had a disability pension and the others were supported by relatives (Tables 2 and 3). About 32.1% of patients were with drug addiction and 18.3% of patients were with alcohol addiction. Six individuals were homeless.
| Age | %(n) | 22-33 | 34-39 | 40-49 | 50-55 | >59 |
|---|---|---|---|---|---|---|
| Male | 64.5(243) | 2.5(6) | 73.3(178) | 21.0(51) | 2.5 (6) | 0.8 (2) |
| Female | 35.5(134) | 12.7(17) | 54.5(73) | 20.9(28) | 10.4(14) | 1.5 (2) |
| Total | 377 | 6.1 (23) | 66.6(251) | 20.9(79) | 5.3 (20) | 1.1 (4) |
Table 1: Gender and Age structure of patients with TB/HIV co-infection (n=377).
| Mode of transmission | %(n) |
|---|---|
| Heterosexual | 65.5%(247)^ |
| MSM* | 20.9% (9) |
| IDU** | 32.1% (121) |
| Education | |
| Secondary and Secondary-special | 97.6% (368) |
| University | 2.4% (9) |
| Employment status | |
| Regular work | 20.9% (79) |
| No work | 79.1% (298) |
| Regular work | |
| University degree | 8.9% (7) |
| Secondary | 91.1% (72) |
| No work | |
| University degree | 0.7% (2) |
| Secondary | 99.3% (296) |
Table 2: Demographic profile of study population (n=377).
| Patient’s cohort% (n) | 2015 (n=180) | 2016 (n=197) | Total |
|---|---|---|---|
| Regular work | 23.9 (43) | 18.3 (36) | 20.9 (79) |
| No work | 54.4 (98) | 57.9 (114) | 56.2 (212) |
| Disability pension | 21,7 (39) | 23.8 (47) | 22.8 (86) |
| Drug addiction | 37.8 (68) | 26.9 (53) | 32.1 (121) |
| Alcohol addiction | 16.1 (29) | 20.3 (40) | 18.3 (69) |
Table 3: Social status of study population (n=377).
As to the route of HIV transmission 247 (65.5%) individuals were infected through sexual intercourse: 144(59.3%) men and 103(76.9%) women; 9(2.4%) individuals were men who had sex with men (MSM). However, out of those 144 men and out of 103 women who self-reported as been infected via heterosexual route 43(17.4%) individuals were also intravenous drug users (IDUs): 37 men and 6 women.
All 377 HIV/TB patients were at C stage of HIV-infection (stage 4 WHO classifications): C3-56.2% (CD4+cell count was <200 cells/mm3); C2-25.7%; C1-18.0%.
Out of 377 individuals with co-infection HIV/TB, there were 214(56.8%) patients with newly diagnosed TB. About 30% (116) of individuals with newly diagnosed TB did not know they were HIV-positive and attended the clinic for TB treatment. Both among newly diagnosed HIV positive patients and the HIV/TB patients registered in specialized care centers the most predominant TB-form was disseminated pulmonary tuberculosis in the phase of infiltration decay-50.5% and 49.7%, respectively (Table 4). Infiltrative TB was detected almost with the same frequency (21.9% and 22.1%, respectively) in both groups of patients. However, TB of intrathoracic lymph nodes was 1.7 higher (p<0.03) in newly diagnosed patients. The other clinical forms were observed in a small percentage of cases (1.9% and 1.2%<respectively). Cavities in the lungs were revealed in 34.3% of newly diagnosed patients and 35.8% of registered patients (Table 5). The rate of active TB (MbT+) among new TB/HIV cases was 49.1% and 44.3% among registered TB cases. In newly diagnosed TB cases multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) were detected in 29.4% of patients. In HIV-positive patients with registered TB, that figure was 40.5%. The most common opportunistic infection was viral hepatitis C/B – 76.9%, followed by Candidiasis–69.5% (Figure 1). A large number of patients had several comorbidities: 45.2% had 2 comorbidities; 17.4%-3; 7.2%-4.
| TB Clinical forms | HIV+patients with newly diagnosed TB %(n) (n=214) | Registered HIV/TB patients*%(n) (n=163) |
|---|---|---|
| Infiltrative TB | 21.9 (47) | 22.1 (36) |
| Disseminated pulmonary TB | 50.5 (108) | 49.7 (81) |
| TB of intrathoracic lymph nodes | 19.6 (42) | 11.7 (19) |
| Focal TB | 5.1 (11) | 4.9 (8) |
| Miliary TB | ---------- | 6.1 (10) |
| Fibrous-cavernous pulmonary TB | ---------- | 3.1 (5) |
| Caseous pneumonia | ---------- | 1.2 (2) |
| Tuberculoma | 0.5 (1) | 0.6 (1) |
| Others | 1.9 (4) | 1.2 (2) |
Table 4: Characteristic of TB clinical forms of patients with HIV/TB co-infection.
| 2015 | 2016 | |||
|---|---|---|---|---|
| New TB/HIV patients%(n) (n=108) | Registered TB/HIV patients%(n) (n=72) | New TB/HIV patients%(n) (n=106) | Registered TB/HIV patients%(n) (n=91) | |
| Cavities | 12.0 (13) | 22.2 (16) | 22.6 (24) | 24.2 (22) |
| MbT+ | 46.3 (50) | 23.6 (17) | 51.9 (55) | 32.9 (30) |
| MDR-TB* and XDR-TB** | 24.1 (26) | 27.8 (20) | 34.9 (37) | 50.5 (46) |
Table 5: Detection rate of cavities, MbT+ and drug resistant TB in patients with HIV/TB co-infection.
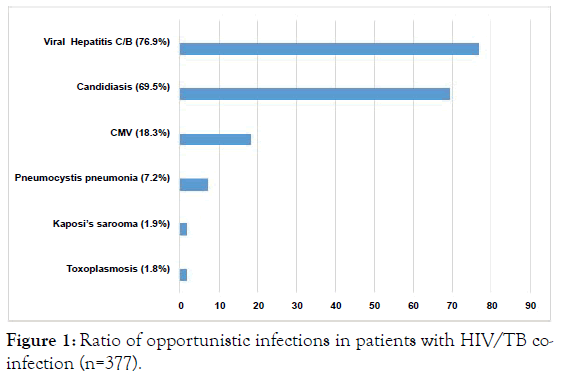
Figure 1. Ratio of opportunistic infections in patients with HIV/TB coinfection (n=377).
Out of 214 individuals newly diagnosed with TB 30.8% (116) were not aware of their HIV-positive status and attended the hospital for TB treatment. Of those 163 individuals who were previously diagnosed with HIV/TB co-infection only 45.4% were steadily on HAART. The other 83 patients (50.9%) took medication sporadically (stopped taking medications 2-3 months after been diagnosed with HIV) and 6 patients (3.7%) refused to undergo treatment. Out of 116 patients with newly diagnosed HIV/TB co-infection 7 (6.0%) refused from HAART.
As to the treatment’s duration, 26.3% (99) of patients were treated at the hospital for up to 2 months or less. Those patients were discharged either for violation of the hospital regime (alcohol abuse/using drugs -52.5% (52 patients)) or for unauthorized departure from the hospital -39.4% (39 patients). Eight patients (8.1%) refused to continue treatment at the hospital. About 20.9% (79) of patients after staying at the hospital for 2-3 months decided to interrupt their treatment. And 56.8% (199) of patients stayed at the hospital for more than 4 months. As to the treatment effectiveness cessation of the M. tuberculosis allocation was 75.2% (79) in newly diagnosed TB patients and 55.3% (26) in registered patients (Table 6). Meanwhile, the percentage of cessation of the M. tuberculosis allocation among patients with MDR-TB and XDR-TB was 79.2% in newly diagnosed and 36.8% in registered patients. Cavity closure had occurred in 54.1% (20) in patients with newly diagnosed TB and 34.2% (13) in registered patients. Those indicators for patients with MDR-TB and XDR-TB in the respective groups were 63.6% and 21.6%.
| Newly diagnosed | Registered | ||
|---|---|---|---|
| MbT+ | Cessation of the M. tuberculosis allocation | MbT+ | Cessation of the M. tuberculosis allocation |
| Total (n=105) | 75.2 (79) | Total (n=47) | 55.3 (26) |
| Total MDR- TB/XDR-TB (n=63): MbT+ (53) |
79.2 (42) | MDR-TB/XDR-TB (n=66): MbT+ (n=38) |
36.8 (14) |
| CV+ | Cavity closure | CV+ | Cavity closure |
| Total (n=37) | 54.1 (20) | Total (n=38) | 34.2 (13) |
| MDR-TB/XDR-TB (n=63): CV+ (11) | 63.6 (7) | MDR-TB/XDR-TB (n=66): CV+ (n=37) | 21.6 (8) |
Table 6: Treatment effectiveness in patients with HIV/TB co-infection.
The mortality rate among patients with HIV/TB co-infection was 17.8% (Table 7). Of those 10.8% accounted for newly diagnosed TB patients. In most of the deceased patients, the main cause of death was progressive tuberculosis and its complications. The immediate cause of death was pulmonary heart failure, cerebral edema and pulmonary edema.
| Cause of death | 2015 | 2016 | Total%/n |
|---|---|---|---|
| Total number of deaths | 26 | 41 | 17.8 (67) |
| Progression of tuberculosis | 14 | 26 | 59.7 (40) |
| Pneumocystis pneumonia | 1 | 1 | 3.0 (2) |
| Pneumonia of mixed etiology (bacterial, fungal, pneumocystic) | 3 | 4 | 10.4 (7) |
| Meningoencephalitis | 1 | 1 | 3.0 (2) |
| Generalized Mycobacteriosis | 3 | 4 | 10.4 (7) |
| CMV | 2 | 24 | 6.0 (4) |
| Lymphoma lung cancer | 2 | 3 | 7.5 (5) |
Table 7: Structure of lethality cases among patients with HIV/TB coinfection.
At present in Russia, there is the stabilization of the epidemiological situation on tuberculosis with a tendency to its improvement. In 2016 the overall incidence of TB decreased by 7.6% compared to 2015 [18]. However, the epidemiological process is negatively affected by the growing HIV epidemic with an increasing number and proportion of patients with late stages of HIV infection, as well as the increasing proportion of patients with MDR-TB (multidrug-resistant TB) and XDR-TB (extensively drug-resistant TB). Despite the wide access to highly active antiretroviral therapy (HAART) the number of HIV positive patients presenting late for care in Russia tends to increase over the past few years: 11.3% in the 2010-year vs. 20.9% in the 2016 year [18]. Our findings show that 40.3% of patients with TB/ HIV who attended the Hospital were with advanced HIV disease (median CD4 count 62 cells/mL). This explains the high rate (50.1%) of patients with disseminated pulmonary TB as it is well known that a significant decrease in the number of CD4 cells favors the development of this TB form.
Due to the social significance of preventing the spread of tuberculosis infection in society one of the most important indicators for the treatment effectiveness of respiratory TB is the cessation of the allocation of M. tuberculosis in the patient. For the period 2015-2016, the percentage of cessation of bacterial excretion was 75.2% in newly diagnosed patients and 55.3% in registered patients. The obtained data corresponds to the general knowledge, according to which the cessation of bacterial excretion in registered patients occurs less frequently than in newly diagnosed patients because the registered patients are characterized by more common tuberculosis processes. Besides, treatment effectiveness in patients is particularly affected by the presence of MDR-TB and XDR-TB. Such patients accounted for 29.4% of all newly diagnosed patients and 40.5% of registered patients. This explains the lower treatment effectiveness of registered patients regarding the cessation of bacterial excretion. In 2016 there was a 2-fold increase of XDR-TB in registered patients comparing with the 2015 year.
Another important indicator of the treatment effectiveness of a patient with respiratory tuberculosis is the closure of the destruction cavities in the lungs. This indicator demonstrates the depth and persistence of the process of healing from tuberculosis. The closure of cavities was 54.1% in newly diagnosed TB patients and 34.2% in registered. Again this indicator reflects a wellknown pattern: the longer there is a cavity of destruction, the more difficult it is amenable to conservative treatment.
At the same time, it should be borne in mind that the results of treatment also depend on the duration of therapy. The length of stay in the hospital for more than 4 months is due to the prevalence of tuberculosis with multiple drug resistance and poor tolerability of anti-tuberculosis therapy, as well as the presence of severe comorbidities. However, our findings showed that about 26.3% of patients were treated in the hospital for less than 2 months and 20.9% left the hospital after 2-3 months of treatment. Analysis of the social characteristics of our patients showed that in most cases, those were socially maladapted individuals, many of whom abused alcohol and used drugs. They live on casual earnings or depend on relatives. In such patients, the motivation for treatment is reduced, many of them are not able to observe the long-term treatment regime of the hospital. All this leads to early discontinuation of inpatient treatment, which dramatically reduces the effectiveness of treatment.
Analysis of mortality rates showed that the fatal outcome was observed in patients suffering from disseminated forms of TB in the late stages of HIV infection that lead to progression and worsening of the functional reserves of the organism, with the accession of opportunistic infections. The main proportion of those patients (10.8%) was TB/HIV patients with primary multiple lesions of several organs and systems, primarily meningoencephalitis of a specific nature. The high mortality rate among TB/HIV patients was because the patients were in the terminal stage of HIV-infection.
An alarming fact revealed in our study was that 45.8% of HIV-positive patients with newly diagnosed TB before being diagnosed with TB were known to be infected with HIV. There are well-established data that HIV enhances the risk of developing tuberculosis shortly after HIV infection [4,20]. Although in Russia there is an extensive network of TB health facilities which include 56 specialized Medical Centers, 144 specialized Medical Care hospitals and 1981 TB Control offices, it seems there is still some discrepancy in TB and HIV management. This data is confirmed by the Efsen et al. and Mansfield et al. surveys conducted among patients with TB/HIV co-infection in Eastern Europe countries which showed poor coordination between TB and HIV health services [21,22].
Also, the discouraging fact was that 65.1% of patients who knew about their HIV positive status did not undergo HAART. As in Russia, the TB treatment, as well as the antiretroviral therapy, is free for all patients the cost of TB clinic and HAART treatment was not a decisive factor. Despite the wide educational campaign in Medical Centers and through media (internet, brochures) about HIV-infection one of the reasons the patients refused to undergo treatment was the belief that the antiretroviral drugs would negatively affect their health and lifestyle. No explanations of the doctors could dissuade them. Some of the patients did not take seriously their illness and some just did not explain their refusal. The study that we carried out previously regarding the knowledge about HIV/AIDS among people living with HIV showed that misconceptions about HIV-infection were characteristic solely for respondents with secondary level education. And that was consistent with the results of studies conducted in other countries [23-26]. Moreover, there are data that education level could be largely considered as a risk factor for active TB among HIVpositive individuals [27,28]. Taken that into the consideration the patients’ late attendance to the hospital and their negligence to their health could be explained by the fact that the majority of individuals in our study had low socioeconomic status: 97.6% had secondary and secondary-special education, and only 22.5% of those had regular work.
It is noteworthy that there were a higher percentage of female patients with TB/HIV co-infection in the age groups 22-33 years and 50-55 years compared with the same male age groups. This may be partly to the fact that women tend to seek medical help more often than men. Especially when it comes to the young men of age 22-33 years who are usually not paying enough attention to their health. As for the older men they often write off their ailments by age. Our findings showed that older men were more likely to present late for medical care [29]. This data is consistent with the findings of other investigators [30-33]. At the same time, several studies did demonstrate that TB frequency was higher in females aged less than 30 years [34-36]. However, it is still unknown whether this is due to behavioral risk factors, biological reasons or treatment history. But surely this aspect should be studied in more detail, on a cohort of patients with the same number of women and men. This further study will allow drawing reasonable conclusions about whether these female age groups are at higher risk for acquiring TB.
Our study thus revealed that the majority of patients with TB/ HIV co-infection were maladapted individuals, many of whom abused alcohol (32.1%) and used drugs (18.3%). For those patients was characteristic the significantly reduced motivation for treatment. The treatment effectiveness cessation of the M. tuberculosis allocation was 75.2% in newly diagnosed TB patients and 55.3% in registered patients. Cavity closure had occurred in 54.1% in patients with newly diagnosed TB and 34.2% in registered patients. The results of the therapy were largely determined by the low adherence of patients to treatment – in the total 48.9% of patients were discharged after 2-3 months/or less of their stay in the hospital- and by the high rate of MDR-TB/ XDR-TB (34.2%). The patients' categorical refusal to undergo medical treatment (4.5%) or discontinuation of the HAART shortly after been diagnosed with HIV (50.9%) remains a serious problem and demonstrates poor knowledge about the importance of earlier access to HIV/TB treatment. Evidently there are some aspects of HIV/AIDS which still need clarification through more detailed and specific HIV/TB educational programmes targeting all population groups and aimed at increasing the interest of the population in maintaining their health.
The fact that there were 56.8% HIV-infected patients with newly diagnosed TB indicates insufficient effectiveness of programmes for early TB testing. Also alarming is the fact that about 30.8% of individuals with newly diagnosed TB were not aware of their HIV positive status. The reinforcement of TB diagnosis is necessary among HIV-infected individuals as well as the better interaction between TB and HIV services.
The informed consent to the processing of personal data was obtained from each patient. The collected data were subsequently depersonalized and as a result there were no identifiable patients’ data. The study was approved by the Ethical Review Board of I.I. Mechnikov Research Institute of Vaccines and Sera.
The authors declare that they have no conflict of interest.
Part of the data was presented at the 12th World Congress on Virology, October 16-17, 2017 Baltimore, USA.
Citation: Nosik M, Rymanova I, Sevostyanihin S, Ryzhov K, Sobkin A (2019) HIV-Associated Tuberculosis in Russia: Results of the Retrospective Cohort Study over the Period of Two Years. J Antivir Antiretrovir. 11:189. DOI: 10.35248/1948-5964.19.11.189
Received: 21-Oct-2019 Accepted: 04-Nov-2019 Published: 11-Nov-2019
Copyright: © 2019 Nossik M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.