
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Review Article - (2020)Volume 11, Issue 8
Upper airway obstruction during sedation can result from changes in either the passive structural properties of the pharynx or from disturbances in neuromuscular control. The maintenance of mechanical upper airway properties may significantly contribute to upper airway patency. Therefore, understanding the pathogenesis of upper airway obstruction may help to establish methods of intervention during sedation. This review summarizes recent literature leading understanding of the implications of changes in upper airway patency by mechanical interventions and respiratory management during sedation.
Recently published literature is focusing on the upper airway characteristic under sedation such as propofol, dexmedetomidine, midazolam and ketamine. Furthermore, effective combination of interventions of postural change and airway management with device including nasal high flow seems to have significant benefits for patients with comfort and safe airway management.
It is important to understand the pathophysiology of upper airway obstruction during sedation and to establish effective interventions based on obtained clinical data to achieve the best-personalized sedation method with safer and more stable conditions.
Upper airway patency; Procedural sedation; Mechanical interventions
Sedation is used in many medical procedures, particularly those that are painful or somewhat invasive. However, compared to general endotracheal anesthesia, sedation management can be challenging to keep the patient spontaneous breathing. This review reassesses the current understanding of the implications of changes in upper airway patency by mechanical interventions and respiratory management such as nasal high flow therapy during sedation.
The importance of understanding the mechanism of upper airway patency during sedation
The upper respiratory tract is a complex system with numerous critical roles (e.g., swallowing, vocalization, maintaining airway patency, and prevention of aspiration). However, anesthetics and sedatives interferes with these functions, and the anesthesiologist must understand how to provide sedation while maintaining the patency of the upper airway and spontaneous breathing. Anesthetic agents, Even in patients whose airways are naturally open when they are awake or sleeping, upper airway obstruction can occur during general anesthesia due to aesthetic suppression of the respiratory center, decreased peripheral muscle activity, increased alertness threshold (decrease in the consciousness level), and decreased sensitivity of negative pressure detection receptors in the pharyngeal airway mucosa. In other words, since various compensatory mechanisms are suppressed during sedation, the patency of the upper airway becomes dependent on morphological factors and is more susceptible to patient-specific, surgical, or procedural risks. During sedation, the nerve reflex is weakened due to the deterioration and loss of consciousness, and the activity of the upper airway dilating muscles is decreased. The upper airway patency becomes dependent on anatomical factors of the upper respiratory tract, and when the upper airway lumen is relatively narrow, partial obstruction is more likely to occur (Figure 1).
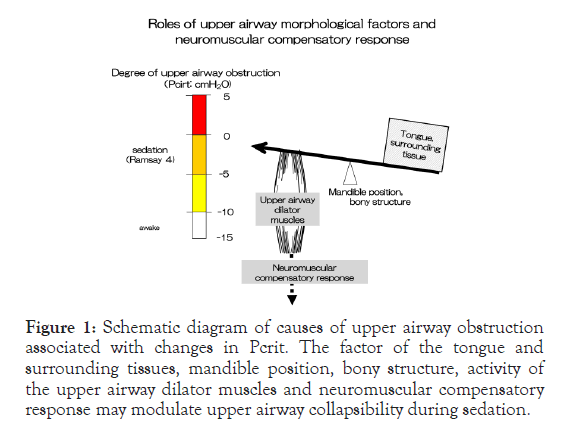
Figure 1. Schematic diagram of causes of upper airway obstruction associated with changes in Pcrit. The factor of the tongue and surrounding tissues, mandible position, bony structure, activity of the upper airway dilator muscles and neuromuscular compensatory response may modulate upper airway collapsibility during sedation.
Quantitative evaluation of upper airway patency
As mentioned above, the anatomical features of the upper airway resemble a structure called a Starling resistor, and the pressure in the upper airway lumen is easily affected by the pressure of the surrounding tissue. The upper respiratory tract is structurally composed of a collapsible segment (pharynx) between two rigid tubes (the nose and trachea). Airflow through the collapsible soft tissue is subject to three different states, depending on how its upstream and downstream pressures are related to ambient (soft tissue) pressure. The upstream pressure at which the respiratory flow stops is called the critical closing pressure (Critical Closing Pressure=Pcrit). The critical closing pressure (Pcrit) is evaluated by analyzing the pressure-flow curve of the nasal cavity pressure (oral pressure) and the actual maximum inspiratory flow in the presence of inspiratory flow limitation. Due to its simple measurement procedure and analysis method, Pcrit is used in many anesthesia- based studies.
Differences in the effect of anesthetics on upper airway patency
Upper airway patency during sedation varies depending on the anesthetic used, such as intravenous anesthetics and sedatives (Table 1). Many quantitative analyses of upper airway patency have been performed with midazolam [1] and propofol [2], which are the most frequently used drugs in anesthesia management under spontaneous breathing. The effects of midazolam and propofol anesthesia on the patency of the upper respiratory tract are similar based on the evaluation of morphological factors using Pcrit as an index. Our study also showed that at an anesthesia depth of BIS40〜60, the Pcrit value for midazolam anesthesia is approximately -5.1 cmH2O [1], and the Pcrit for propofol anesthesia is approximately -4.4 cm H2O [2]. This confirms that the effects on the morphological factors are similar. Moreover, since the value of Pcrit during anesthesia is almost similar to that (-4.5 cm H2O) [3] during the rapid eye movement (REM) stage of natural sleep, upper airway patency during anesthesia under spontaneous breathing may be similar to spontaneous sleep. The most recent study that compared the upper respiratory patency during non-Rapid eye movement (NREM) natural sleep (Pcrit=-2.0 cm H2O: mean) with propofol sedation (Pcrit=2.1 cm H2O: mean) found that natural sleep had a higher degree of passive patency, and concluded that a detailed respiratory physiologic analysis should be conducted in the future [4]. Regarding the effect of sedatives on upper airway patency, similar upper airway patency of propofol (Pcrit=-0.6 cm H2O: median) and dexmedetomidine (Pcrit=0.3 cm H2O: median) was recently reported [5], which is in line with the present review findings. Furthermore, in recent years, many methods using ketamine in combination with sedation for invasive procedures have been reported, mainly in Europe. In our study, ketamine (Pcrit=-0.08 cm H2O) had similar static upper airway patency as propofol (Pcrit=-0.32 cm H2O) and dexmedetomidine (Pcrit=-0.28 cm H2O), reflecting the anatomical features. The airflow resistance of the upper respiratory tract, which is considered to reflect the muscle activity of the upper airway dilating muscle, was revealed to be higher with ketamine than with propofol and dexmedetomidine. A larger compensatory defense mechanism against partial upper airway obstruction was shown [6]. Together, these findings are consistent with those suggested in previous studies of rats [7] that suggested that Ketamine has a stronger biological defense mechanism against upper airway obstruction than other sedatives due to the increased muscle activity of the upper airway dilating muscle during sedation. This supports the efficacy of the ketamine combination in the current sedation method and suggests the development of a new sedation method in the future.
| Drug | Level of sedation | Pcrit (cm H2O) | Reference |
|---|---|---|---|
| Propofol | BIS 65 ± 13, OAA/S 2, RASS score -4 | -0.6 | Lodenius, A. et al. 2019. Anesthesiology |
| 50<BIS <70 | -0.32 | Mishima, G. et al. 2020. Physiological Reports | |
| 40<BIS <60 | -4.4 | Hoshino, Y. et al. 2009. Respiratory Physiology & Neurobiology | |
| BIS <50 | -2.0 ~ -1.9 | Maddison, KJ. Et al. 2020. Respiration and Sleep Medicine | |
| Midazolam | 40<BIS <60 | -5.1 | Ayuse, T. et al. 2009. Anesthesia Analgesia |
| Dexmedetomidine | OAA/S score 2~3, RASS score -3 | -0.28 | Mishima, G. et al. 2020. Physiological Reports |
| BIS 74 ± 13, OAA/S 3, RASS score -2 | 0.3 | Lodenius, A. Et al. 2019. Anesthesiology | |
| Ketamine | OAA/S score 2~3, RASS score -3 | -0.08 | Mishima, G. et al. 2020. Physiological Reports |
| Sleep | REM | -4.5 | Patil, Sp. Et al. 2007. Journal of Applied Physiology |
| non-REM, REM with temazepam | 1.6~2.1 | Maddison, KJ. et al. 2020. Respiration and Sleep Medicine |
Table 1: Differences in the effect of anesthetics on upper airway patnecy based on Pcrit evaluation in comparison with sleep stage.
Neuromuscular compensatory response during sedation enhances the activity of the upper airway dilating muscle For persistent upper airway obstruction, activation of the upper airway dilating muscle from nerve or muscle compensation without wakefulness occurs during sleep. During propofol anesthesia under spontaneous breathing, a part of the nerve or muscle compensatory reaction without arousal remains [2]. More interestingly, an analysis of upper airway patency in males and females under midazolam anesthesia revealed a sex difference in the nerve and muscle compensation response [1]. In patients with obstructive sleep apnea, this compensatory function is known to be ineffective [3]. The wakefulness response is crucial when managing anesthesia under spontaneous breathing in such patients.
Prevention and treatment of upper airway obstruction during sedation
A study showed that changes in head and upper body positions effectively maintain the patency of the upper respiratory tract. Lateral and Fowler positions are better than the supine position in maintaining upper airway patency [8]. Position selection during anesthesia management under spontaneous breathing and after surgery is also important. Kobayashi and colleagues investigated the effect of head elevation during propofol anesthesia under spontaneous breathing. They confirmed that Pcrit decreased by 4.4 cm H2O in response to a 6 cm head rise despite cervical anteflexion during head elevation. They estimated that a head elevation of approximately 7 cm is the most effective [9]. Ikeda and colleagues [8] confirmed that 30° body elevation during midazolam anesthesia reduced Pcrit by approximately 5.4 cm H2O, and was effective at maintaining upper airway patency. The application of a newly developed semi-automatic control airway dilating device was also shown to be effective [10,11]. In addition, some studies have examined airway dilation due to changes in the position of the mandible and head elevation/retroflexion. A method that involves releasing the airway obstruction by improving the position of the jaw has also been shown to be effective. Kurata and colleagues developed a method of opening the airway by moving the mandible forward with an air-actuated actuator (artificial muscle) [10]. Furthermore, Ishizaka and colleagues developed an airbag type semi-automatic head elevation method and confirmed its effectiveness against airway obstruction during sedation [11].
Intervention with nasal high flow
The effects of a device called nasal high flow therapy (NHF) that inhales humidified air at a high flow rate to manage breathing during sedation are being studied. The mild positive pressure load (3~6 cm H2O), washout of exhaled CO2, and reduction of rebreathing help to improve respiratory function and have been widely applied to prevent hypoxemia [12-16]. It has also been indicated that nasal high flow might be alternative intervention for maintaining upper airway patency during sedation [17,18]. However, in a recent study, an air-only NHF loader (trade name AIRVO®) that does not use oxygen was shown to be useful during moderate sedation using intravenous anesthesia with propofol as a respiratory control method under spontaneous breathing. Reducing the respiratory effort can help maintain CO2 concentrations and oxygen saturation within the normal range [19]. Therefore, in addition to respiratory control during sedation, the effect of anesthesia during the acute postoperative phase persists. NHF without oxygen may be effective in patients with somnolence or sedation. If NHF reduces breathing effort during sedation with propofol enabling patients to breathe more easily, we believe that this will help to compensate for various risk factors of upper airway obstruction during dental and oral surgical procedures and maintain upper airway patency. Furthermore, saliva can be easily swallowed with an NHF, and its future clinical application is expected [20]. NHF may also be effective in preventing hypercapnia and hypoxemia during endoscopic retrograde cholangiopancreatography and endoscopic submucosal dissection under sedation. Currently, we are conducting a randomized controlled trial [21,22] with patients undergoing oral surgery. We are aiming to determine whether wearing NHF during surgery helps to preventearly postoperative hypoxemia and hypercapnia after surgery [23]. Causes of postoperative early hypoxemia and hypercapnia include respiratory depression and upper airway obstruction due to the residual effects of anesthetics, soft-tissue edema in the surgical field, as well as atelectasis and decreased respiratory function from surgery. Routine oxygen administration for several hours after general anesthesia should help prevent early postoperative hypoxemia. Patients undergoing surgery under general anesthesia are often given oxygen via a nasal cannula or face mask to prevent hypoxemia during the acute postoperative phase. The face mask is adjusted so that the FIO2 concentration is approximately 0.4, with an oxygen flow rate of 5-6 L/min. This helps to control breathing so that SpO2 value normalizes. However, respiratory management in the acute postoperative phase is essential to maintain normal respiratory function, including regulation of inspiration and expiration. Therefore, it is important not to merely maintain SpO2 but also monitor the level of exhaled CO2. Furthermore, the administration of a high oxygen concentration to patients with chronic respiratory disease and sleep apnea should be performed carefully. If oxygen concentration is increased when SpO2 is low, CO2 narcosis may cause a consciousness disorder due to hypercapnia. The consciousness level may be lowered, and secondary complications may occur. Furthermore, in a recent study by Liao P et al. [24] on postoperative oxygen therapy in surgical patients with obstructive sleep apnea, a significant increase of PtcCO2 was found in 11.4% of patients, especially those receiving oxygen on postoperative night 1.
Saliva swallowing management during sedation Saliva swallowing management during sedation
It has been well recognized that sedative drugs, such as midazolam, propofol and dexmedetomidine, depress physiological function of saliva swallowing reflex [25,26]. Recently we have postulated that dexmedetomidine sedation might depress saliva swallowing due to inhibition of larynx movement [25]. It should be noted that the maintenance of both saliva swallowing function and upper airway patency can be accomplished within very narrow range of physiological control system during sedation [20]. If we want to keep airway open by mandibular advancement, we may lose ability to swallow saliva during any type of sedation, including dexmedetomidine sedation that is believed to be similar to natural sleep stage. Recently, Hanamoto et al. suggested that mandibular advancement impairs swallowing ability more than head extension [27]. We have indicated that mandible re-positioning may strongly influence the coordination between nasal breathing and non- nutritive swallowing by altering respiratory parameters and by inhibiting movement of the tongue-jaw complex [28]. Although previous study suggested that mandibular advancement as well as nasal high flow may reduce degree and duration of hypoxemia due to prevention of airway obstruction, there might be another risk factor of inhibition of saliva swallowing [17]. More interestingly, the application of nasal high flow during sedation may enhance swallowing function with increasing levels of NHF by reducing the latency of the reflex [20]. It has been aware that the secretion rate of saliva may decrease during sedation similar to sleep stage in order to reduce amount of saliva aspiration. However, if surgical procedure requires water supply, for example of dental treatment or oral surgery, the accumulated water could be major risk factor to cause saliva aspiration by influx into airway tract during sedation under depression of swallowing function.
Mechanical upper airway anatomy may become the dominant factor governing upper airway collapsibility during sedation due to the significant impairment of neural mechanisms controlling compensatory neuromuscular responses. It is therefore important to understand the effectiveness of mechanical interventions and develop a systematic approach in evaluating critical factors that contribute to the maintenance of upper airway patency during sedation.
Citation: Kurata S, Mishima G, Sanuki T, Ayuse T (2020) How to Manage Upper Airway Patency during Procedural Sedation. J Anesth Clin Res. 11: 962. DOI: 10.35248/2155-6148.20.11.962.
Received: 13-Aug-2020 Accepted: 21-Aug-2020 Published: 28-Aug-2020 , DOI: 10.35248/2155-6148.20.11.962
Copyright: © 2020 Kurata S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.