Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2022)Volume 12, Issue 2
Traditional analytical approaches of HPLC found to be insufficient for solving upcoming challenges in species specificity and sensitivity analysis. Modern analytical hybrid techniques which are referred as hyphenations have been introduced from the traditional techniques to overcome the challenges in the analysis of components. Hyphenated approaches combine separation and detection procedures through the use of chromatographic and spectroscopic techniques. The various hyphenations of that are developed until now are LC-MS, LC-NMR, LC-IR, LC-DAD and it also includes multi hyphenations like LC-MS-MS, LC-ESI-MS. These techniques show high specificity and sensitivity. Now-a-days various types of LC-MS systems with various types of interfaces are a commercially available. The application of these hyphenations in many sectors such as forensic science, environment, biotechnology, pharmaceutical field is discussed.
Hyphenation; LC-MS; LC-NMR; LC-IR; LC-DAD; LC-MS-MS; LC-ESI-MS
Hirschfield invented the term “Hyphenation” in 1980 to express the possibility of combining two or more instrumental analysis methods in single run. When compared to a single analytical technique, the primary goal of this coupling is to generate an information rich detection that can be used for both identification and quantification” (Figure 1) [1].
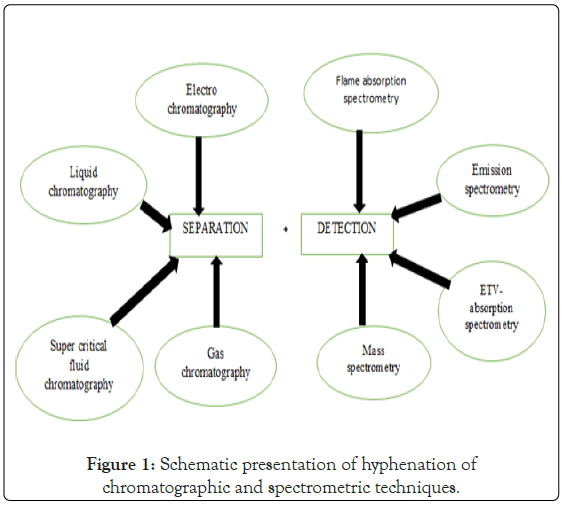
Figure 1: Schematic presentation of hyphenation of chromatographic and spectrometric techniques.
With the use of an appropriate interface, a hyphenated procedure combines or couples two different analytical procedures. Mainly chromatographic techniques are combined with spectroscopic techniques [2]. Chromatography yields pure or very pure fractions of chemical components in a mixture, while spectroscopy yields selective information for component identification. A hyphenated approach will consist of a combination of a separation technique with on-line spectroscopic detection technology [3]. Hyphenated techniques have received an increasing amount of attention in recent years as a primary means of resolving complex analytical problems [4]. The various hyphenation techniques of HPLC are listed below [5].
Double hyphenated techniques
• LC-MS
• LC-NMR
• LC-IR
• LC-DAD
Multi hyphenated techniques
• LC-MS-MS
• LC-ESI-MS
LC is an effective and frequently used approach for separating mixtures into their constituent parts. IR spectroscopy is incredibly useful for identifying functional groups and has good compound-identification capabilities, making it incredibly well adapted to structural isomer variation [4].
LC-IR or HPLC-IR is a hyphenated approach that was developed by combining LC with the detection method Infrared Spectrometry (IR) or FTIR. While HPLC is one of the most efficient separation technologies available today, Infrared Spectroscopy (IR or FTIR) is a useful spectroscopic technique for identifying organic compounds because the structures of organic compounds have many absorption bands that are characteristic of specific functionalities, such as -OH, -COOH, and so on, in the mid-IR region [3]. Combining HPLC and IR, on the other hand, is challenging, and progress in this hyphenated technique is slow since the mobile phase solvent's 237 absorption bands are so broad in the mid-IR region that they frequently block the sample components' small signal.
Since FT-IR is an absorbance technique, the shape of the sample throughout the measurement procedure is critical. For a particular mass or volume of analyte, reducing the diameter by a factor of two leads in a deposit with four times the thickness and four times the optical density. Because the IR detector is restricted by total light, a two-fold reduction in deposit diameter improves the signal-to-noise ratio by four times. As a result, for the LC-IR hyphenation process to generate an instrument that yields entire mid-infrared spectra, it must:
• Remove the solvent without overextending the vacuum system with diluent gas or causing thermal damage to the analysis.
• Ensure that analyses are sent to the spectrometer as early as possible.
• In a thick deposit carryout FTIR analysis.
• Maintain chromatographic resolution [2].
Instrumentation and working
In most of the LC-IR processes, the solvent elimination strategy is chosen, the mobile phase solvent is removed, and IR detection is performed in a medium that is transparent to IR light [6]. The collection of sample components in the eluent is usually done with KBr or KCl salts and heating up the medium before IR detection removes the volatile mobile phase solvents (Figure 2). For the solvent elimination method, there are two types of interfaces:
Figure 2: LC-IR system.
• Diffuse-Reflectance Infrared Fourier Transform (DRIFT) approach.
• Buffer-memory technique [7].
Applications
• Analysis of characterization, functionality and degradation.
• Compositional analysis of copolymers across molecular weight distribution.
• High temp GPC-IR analysis of polyolefin copolymer branching.
• Polymer blend ratio analysis across the molecular distribution.
• Polymer additive and impurity analysis.
• Competitive analysis for de-formulation of polymers and additives.
• Controlling and optimising processes.
• Coating, adhesive, sealant and elastomer reactive polymer analysis.
• Chemicals, forensics and pharmaceuticals isomer analysis [6].
The hyphenated techniques LC-MS or HPLC-MS relate to the integration of LC (Liquid Chromatography) separation capabilities with Mass Spectrometer (MS) mass analysis capabilities. Chromatography-MS coupling is a well-established chemical analysis method that dates back to the 1950s. Because more than 85 percent of natural chemical compounds are polar and thermally labile, and GC-MS cannot handle these samples, LC-MS has become one of the most extensively used chemical analysis techniques. HPLC-MS, for example, is the most widely used analytical technology in proteomics and pharmaceutical laboratories. Food, insecticides, and plant phenols are some of the other key applications of LC-MS.
The combination of MS and LC systems is appealing because liquid chromatography can separate delicate and complicated natural mixtures with well-defined chemical compositions (e.g. biological fluids, environmental samples, and drugs. While liquid chromatography separates mixtures with several components, mass spectrometry can identify individual component structural identity with high molecular specificity and detection sensitivity [8]. LC-MS may be applied in variety of industries like biotechnology, environment monitoring, food processing and pharmaceutical, agrochemical, and cosmetics [7]. Identification of components in a mixture can be accomplished using a variety of ways. When it comes to structural identification, LC-MS is the preferred method.
Instrumentation and working
From Figure 3 the instrumentation of LC-MS comprises of the following parts [9]
Figure 3: LC-IR system.
• A LC unit.
• An interface between the LC and MS.
• An ion source that ionizes samples (e.g., API unit).
• An ion guide (an electrostatic lens that efficiently introduces the generated ions into the MS) [4].
• A mass analyser unit that separates the ions based on their mass-to-charge (m/z).
• A detector unit that detects the separated ions.
An LC-MS system includes an interface that efficiently transports separated components from the LC column into the MS ion source, in addition to the liquid chromatography and mass spectrometry instruments. Because the LC and MS devices are inherently incompatible, the interface is required. While the mobile phase in an LC system is a pressurised liquid, MS analysers are often operated at high vacuum (about 10-6 /10-7 Hg). As a result, the eluate from the LC column cannot be directly pumped into the MS source. Overall, the interface is a mechanically simple section of the LC-MS system that transfers the maximum quantity of analyte, removes a considerable portion of the mobile phase utilized in LC and keeps the chromatography products' chemical identities (chemically inert). The interface must not interfere with the MS system's ionising efficiency or vacuum conditions as a minimum requirement [10].
The most often used LC-MS interfaces nowadays are based on Atmospheric Pressure Ionisation (API) techniques such as Electrospray Ionisation (ESI), Atmospheric-Pressure Chemical Ionisation (APCI), and Atmospheric Pressure Photoionization (APPI). After a two-decade research and development process, these interfaces became available in the 1990s [11]. Quadrupoles, Quadrupole ion traps, Time-To-Flight (TOF), Time-To-Flight Reflection (TOFR), and Ion Cyclotron Resonance (ICR) mass analysers are among the mass analysers employed. In this technology mutual signal is supressed resulting in clear mass spectra [12].
Electron multiplier and Micro Channel Plate (MCP) are two typical MS detectors that use the secondary electron emission method. The number of ions that reach the detector is converted to signal intensity and sent to a computer along with the LC chromatogram. In the MS, the ion guide, mass analyser, and detector are all contained in a vacuum. The produced ions can be introduced, processed, and detected in the MS with little collision and loss by keeping it in a vacuum. For a fast survey of natural substances, HPLC linked to UV and Mass Spectrometry (LC-UV-MS) has proven to be particularly useful in conjunction with biological screening.
Applications
• It Provides information on the analyte molecule's molecular weight and fragmentation pattern.
• Assist with analyte molecule identification.
• Using qualitative analysis, an unknown molecule can be reconstructed from MS data.
• It is used to track impurity profiles during pharmaceutical development and scaling up, as well as to assess the safety of batches utilised in clinical trials.
• It has applications in volatile explosive residue analysis.
• It's frequently utilised in the field of bioanalysis, and it's particularly useful in pharmaceutical pharmacokinetic research.
• It's a method for detecting and identifying the components of a complicated mixture in proteomics.
• It is commonly used in drug development because it provides for speedy molecular weight confirmation and structural identification [7,9].
• It is also utilised for the analysis of natural products and the profiling of secondary metabolites in plants.
It is a hyphenated technique that combines High-Performance Liquid Chromatography (HPLC) and Nuclear Magnetic Resonance spectrometers (NMR), they have been applied widely used in the investigation of complicated mixtures including unknown components, such as contaminants and metabolites in pharmaceuticals, natural products and synthetic polymers have been used in a variety of ways it was reported in 1978.
History
Watanabe and Niki performed the first on-line LC-NMR experiments in the late 1970s, demonstrating stopped-flow observations of a mixture of known compounds. Military jet fuel was the first real sample studied by LC–NMR, utilising normal phase columns, deuterated chloroform, and Freon. By delivering the most structural information about plant-derived extracts, NMR has shown to be a standout detector for LC [13]. NMR is a potent analytical method for resolving complicated mixtures with unknown components including contaminants and metabolites in medicines, natural products, and synthetic polymers. Because of the employment of highly magnetic field magnets and very sensitive probes, as well as the maturation of peripheral technologies such as solvent removal technology and automatic measurement software suitable for multicomponent analysis, LC-NMR is the most sensitive approach.
Modes of LC-NMR
• Continuous flow (on flow)-Eluent sampled in “real- time” as flowing through NMR detection coil.
• Stopped flow-Pump is stopped at desired location and data acquired.
• Time Slices-Regions, or “time-slices” of interest are analysed.
• Peak parking-Peaks of interest are “parked” in off-line sample loops [14,15].
• Peak trapping-Solid phase extraction cartridges are used to “re-concentrate” samples.
Instrumentation and working
Generally, from Figure 4 in LC-NMR system the LC unit comprises of following parts.
Figure 4: Typical LC-NMR system.
• Auto sampler
• LC pump
• Column
• Non-NMR detector (e.g., UV, DAD, EC, refractive index, or radioactivity)
This detector directs the flow into the LC-NMR interface, which can be equipped with extra loops for intermediate storing of selected LC peaks. The flow from the LC-NMR interface is then routed to either the flow-cell NMR probe-head or the waste receptacle. After passing through the probe-head, the flow is sent to a fraction collector for recovery and further investigation of the various fractions studied by NMR [2].
The eluent flow is aimed towards the LC-NMR interface. In LC-NMR investigations, reversed phase columns are used, and they use a binary or tertiary solvent mixture with an isocratic or gradient elution method. The presence of a proton in the solvent makes obtaining a satisfactory NMR spectrum challenging. An NMR spectrometer's receiver is unable to distinguish between strong solvent signals and faint material signals at the same time. Solvent signal suppression, which is achieved via soft-pulse repeated irradiation, overcomes this disadvantage [7].
Applications
• Characterization of xenobiotics and endogenous metabolized directly from biological fluids.
• Analysis of crude extracts of natural products and plant-derived chemicals. This technique is designed to identify potential therapeutic candidates in plant products quickly.
• Detection of bulk drug impurities during drug-tability testing-LC-NMR/MS data provides for a complete characterisation of all impurities present in the drug [15].
• Determination of unstable chemicals or compounds produced in situ that are not detectable or isolated using existing methods.
• Drug impurities identification.
• It has its applications in polymer analysis.
It's an analytical technique for determining the purity of an analyte or related impurity peak eluting during a high- performance liquid chromatography separation. Modern high- performance liquid chromatographic separations frequently include a diode array detector, allowing the user to examine the chromatogram of separated chemicals in wavelengths ranging from 190 nm to 900 nm.
Principle
• The absorption in the ultraviolet to visible area is detected by a DAD. While a UV-visible detector has only one sample, side light receiving section, a DAD has multiple photodiode arrays to obtain information over a wide range of wavelengths at one time, which is a benefit of DAD (Figure 5).
Figure 5: The principle of the HPLC-DAD data set.
• The spectra can be measured for every one second by continuous elution during HPLC spectra with continuous elute delivery [16].
• If the measurement is performed to a fixed wavelength, the components are identified solely by their retention time, making identification difficult. If a tiny fluctuation in retention time occurs, the DAD can be used to identify omponents by comparing the spectrum.
• DAD aids in the development of quality by allowing teams to make simpler process decisions in the delivery of incremental and iterative solutions.
Applications
• In the environmental sciences, this method can be used to improve the credibility and reproducibility of analyte identification and quantitative analysis.
• DAD provides analysis of each compound at its highest sensitivity for detection of each component.
• The HPLC-DAD method can be a useful tool for the quantitative and qualitative evaluation of the selected polyphenol compounds can be improved in the future for the examination of the polyphenol of interests in individual herbal infusions [17].
• One of the most used techniques for phenolic compound analysis is HPLC-DAD.
It's a sensitive analytical technique that's been widely used to profile glycoforms at the glycopeptide level. It is a hyphenated mass spectrometry approach that combines the great accuracy of the mass spectrometer with the resolution of HPLC separation. This approach has been utilised to profile glycoforms of more complicated therapeutic proteins having one o-3 Nglycosylation site, such as erythropoietin. LC-ESI CID-MS-MS has recently become popular for glycan sequencing due to its improved speed and sensitivity [18,19].
Application
This equipment can analyse intact proteins, mixtures of proteins and peptides, biomarker investigations, and post-translational modification identification.
It's a technique for analysing tiny compounds in liquids. Liquid chromatography with tandem mass spectrometry is a potent analytical technology that combines liquid chromatography's separating power with triple quadrupole mass spectrometry's high sensitivity and selective mass analysis capability.
LC-MS-MS employs liquid chromatography in the same way as HPLC does. Following chromatographic separation, molecules are ionised with an energy source, and these ionised molecules travel through an array of magnets, which further separates molecules based on the m/e ratio. As a result, LC-MS-MS allows for substantially shorter run durations while also increasing precision and accuracy [2].
Application
Due to its greater specificity over immunoassays, LC-MS-MS for the study of reproductive hormones is becoming more popular.
As a result, it can be concluded that the hyphenated approaches of HPLC are significantly superior and more beneficial than single traditional procedures. The hyphenation approaches include the combination of separation-separation, separation- identification techniques. These hyphenated approaches lead to thorough examination of the components as well as we can analyse the medicine with the help of these approaches.
The author expresses sincere thanks to the School of Pharmaceutical Science and Technology, Jawaharlal Nehru Technological University Kakinada, 533001, East Godavari district, Andhra Pradesh, India.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Navaziya Md (2022) HPLC Hyphenations in Advanced Analytical World. J Phys Chem Biophys. 12:328.
Received: 14-Mar-2022, Manuscript No. JPCB-22-16883; Editor assigned: 17-Mar-2022, Pre QC No. JPCB-22-16883 (PQ); Reviewed: 31-Mar-2022, QC No. JPCB-22-16883; Revised: 06-Apr-2022, Manuscript No. JPCB-22-16883 (R); Published: 13-Apr-2022 , DOI: 10.35248/2161-0487-22.12.328
Copyright: © 2022 Navaziya Md. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.