Journal of Geology & Geophysics
Open Access
ISSN: 2381-8719
ISSN: 2381-8719
Research Article - (2023)Volume 12, Issue 4
Source water physicochemical properties and impacts on water quality were evaluated in volcanic and sedimentary aquifer systems in the study area for management and sustainable development. From hand-dug wells, boreholes, rivers, and springs, fifty (50) samples were taken. The major ions were examined, and physicochemical parameters were measured in-situ. The samples' Electrical Conductivity (EC), Total Hardness (TH), Total Dissolved Solids (TDS), and pH all fell within the WHO-permitted range. Twenty-nine (58%) samples were classified as excellent water quality (rank I) according to the Drinking Water Quality Index (DWQI). At the same time, 13 (26%) samples had drinking water of good quality (rank II). In the research area, the distribution of groundwater physicochemical indicators and Drinking Water Quality Index (DQIQ) was shown. The Ca-Cl type is the most prevalent hydrochemical facies, according to the results of the Piper plot. The rock dominance zone in Gibbs plots contained 86% of the samples and 14 fell in the precipitation dominance zone. According to the Wilcox diagram connecting sodium percentage with total concentration, all of the samples fell in the good to permissible for irrigation. Based on Residual Sodium Carbonate (RSC) and Soluble Sodium Percentage (SSP), the results indicated that the majority of the groundwater samples were suitable for irrigation.
Groundwater; Physicochemical parameters; Water quality index; Wilcox; Piper and Gibbs diagram
Groundwater is a renewable resource that can be used for worldwide water supply. It is found as multilayered aquifers almost everywhere on the earth's surface, including in volcanic areas such as volcanic aquifers, which are an important and sometimes the only source of groundwater in many parts of the world [1]. Many countries around the world, including Cameroon, obtain their groundwater from volcanic sources that are stored in the atmosphere and erupted from volcanic rocks. More than 90% of domestic and industrial water in Cameroon comes from groundwater, mostly extracted from volcanic sources. [2]. Understanding groundwater systems is important because surface waters are becoming increasingly unreliable due to pollution and climate change. Hydrogeologists in urban and rural areas deal with many water-related problems [1,3]. One such industry is groundwater pollution, which is a direct result of changes in agriculture and increased by natural and anthropogenic pollutants [4,5]. Due to the rapid increase in population, cities, industry and agriculture, groundwater is under pressure both in terms of quality and quantity [6]. The hydrogeochemistry of water can be used to determine groundwater quality in any region, as groundwater quality is a major concern due to both geological and anthropogenic factors [7]. In general, the indices are designed to interpret water quality data in an easyto- understand way. A groundwater monitoring and enforcement policy was developed in the study area by measuring groundwater samples and introducing water quality indicators. Irrigation WQI is also used as a method of collecting data and communicating water quality [8]. The presence of soil bacteria is dependent on external bodies such as pond water, animal waste [9], organic fertilizers [10], sewage [11] and chemical fertilizers [12]. Physical and chemical parameters are operational characteristics of groundwater assessment that are affected by natural processes, particularly geological structures and human activities [3,5].
In addition, a number of other elements, including the physical makeup of the vadose zone's constituents, chemical buildup, weathering, dissolution, precipitation, ion exchange, and various biological processes that typically take place below the surface, can affect the water quality. The impact of groundwater contamination is particularly pronounced in rural areas in developing countries such as Cameroon [13]. A number of studies have been conducted using different analytical methods to identify critical groundwater quality conditions in different regions of Cameroon [13-17].
Groundwater quality assessment using hydrochemical methods is of great importance in rural areas. However, a thorough study of groundwater quality in the study area has not been conducted to date. Most people use the water from shallow aquifers, which are prone to contamination, for their daily activities. The study area is a predominantly agricultural locality where the use of pesticides, chemical fertilizers, and poor sanitation facilities make shallow aquifer contamination much easier than contamination of deep groundwater by vertical flow in a downward direction [18].
The goal of this study was to determine the hydrogeochemical characteristics of groundwater sources, implications for groundwater quality, impart of water-rock interactions, and to gauge the extent to which anthropogenic influences have affected the quality of the water sources in the study area. To accomplish this goal, a number of indicators, including the Ground Water Quality Index (GWQI), Residual Sodium Carbonate (RSC), Permeability Index (PI), Kelly's Ratio (KR), Magnesium Hazard (MH), Sodium Percent (Na percent), Sodium Adsorption Ratio (SAR), and Soluble Sodium Percentage (SSP) were computed.
The geographical region and the climate
The study area, located on the eastern slope of Mount Fako in southwestern Cameroon, lies between 4°8'30" and 4º25'0" North latitude and 9º12'0" and 9º28'30" East longitude (Figure 1). Temperatures in this region are generally 20ºC during the wet season and 33ºC during the dry season, reflecting the humid equatorial climate of the region. August is the wettest month with more than 10,000 mm of precipitation during the year is the driest month with an average rainfall of 500 mm and January is the driest month with an average rainfall of 30 mm [19]. Daytime temperatures throughout the year range from 17ºC to 34ºC.
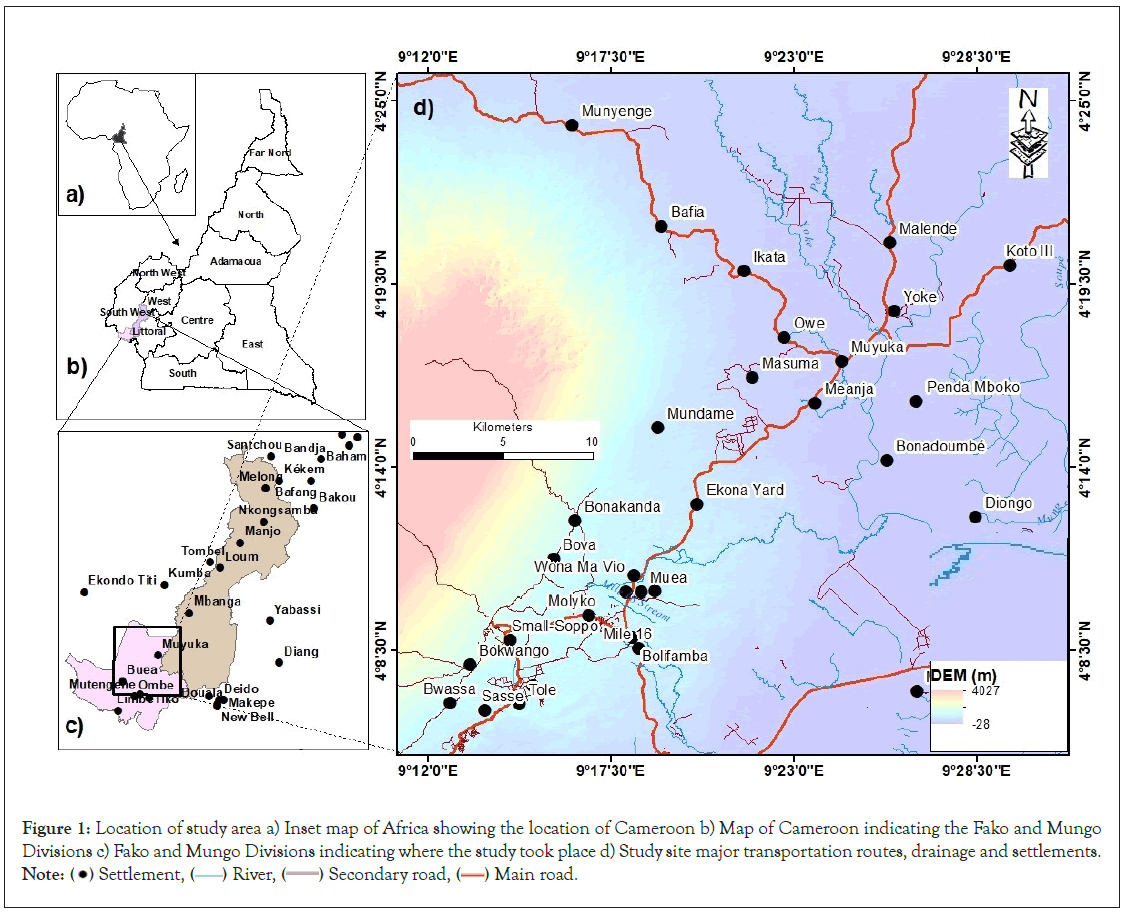
Figure 1: Location of study area a) Inset map of Africa showing the location of Cameroon b) Map of Cameroon indicating the Fako and Mungo
Divisions c) Fako and Mungo Divisions indicating where the study took place d) Study site major transportation routes, drainage and settlements. Note:  Settlement,
Settlement,  River,
River,  Secondary road,
Secondary road,  Main road.
Main road.
Geology and hydrogeology
Geology: Mount Cameroon forms part of the Cameroon Volcanic Line (CVL), an active volcanic belt of 12, other active volcanoes across the country in a SW–NE fashion from the Atlantic Ocean [20]. The general geology of Mount Cameroon is represented by Precambrian metamorphic basement; intruded by gneisses and granite [21]. The volcanic rocks of Mount Cameroon (Figure 2) [18], with the exception of the nephelinite series of Mt. Etinde which are typically alkaline. The massif is underlined Mainly by Ferromagnesian (MAFIC) basaltic and tephra bedrock emanating from a shield Strombo-Hawaian volcanic phenomena [22,23]. Chemical weathering is favored by the high elevation and sea proximity, extreme variable moisture conditions between the seaward and landward flanks of the volcano [24]. The subsoil is composed primarily of Andosal and larger water sources, leading to a dense network of springs and water sources [15]. A combination of intense meteorological processes such as heavy rainfall (second only to Cherrapunji in India), high temperature, humidity, and relatively soluble and variable substrates contribute to chemical weathering, ion exchange and enhanced rock-water interactions. Combined with anthropogenic activity, these processes lead to the emergence of different types of water forming different hydrogeochemical phases (Table 1).
| No. | ID | Towns | Lat | Long | No. | ID | Towns | Lat | Long |
|---|---|---|---|---|---|---|---|---|---|
| 1 | OW1 | Stranger quarter | 4.175 | 9.280 | 26 | OW17 | Camp 3 | 4.298 | 9.462 |
| 2 | OW2 | Malende | 4.321 | 9.428 | 27 | OW18 | Camp2 | 4.278 | 9.449 |
| 3 | RV | Penda mboko | 4.182 | 9.309 | 28 | OW19 | Bone quarter penda mboko | 4.358 | 9.409 |
| 4 | OW3 | Yoke | 4.218 | 9.305 | 29 | CAT2 | Koke | 4.262 | 9.443 |
| 5 | OW4 | Mbo quarter,yoke | 4.170 | 9.307 | 30 | BH2 | Lysoka | 4.285 | 9.401 |
| 6 | OW5 | Malende candef mosack Quarter | 4.355 | 9.315 | 31 | CAT3 | Lyongo | 4.337 | 9.436 |
| 7 | OW6 | Malende | 4.158 | 9.279 | 32 | BH3 | Check point buea | 4.260 | 9.364 |
| 8 | OW7 | Yoke nangi street | 4.157 | 9.249 | 23 | SPG5 | Muea | 4.288 | 9.414 |
| 9 | OW8 | New layout muyuka | 4.301 | 9.378 | 34 | OW20 | Muea | 4.267 | 9.434 |
| 10 | OW9 | Akawuh quarter | 4.158 | 9.292 | 35 | OW21 | Matouke 2 | 4.283 | 9.404 |
| 11 | SPG1 | Kwe kwe | 4.155 | 9.270 | 36 | CAT4 | Mile 18 | 4.310 | 9.427 |
| 12 | OW10 | Kwe kwe | 4.346 | 9.435 | 37 | OW22 | Yoke king bar | 4.285 | 9.403 |
| 13 | SPG2 | Ekona corner road | 4.144 | 9.236 | 38 | BH4 | Buea town | 4.287 | 9.400 |
| 14 | SPG3 | Mautu cross river | 4.333 | 9.435 | 39 | OW23 | Muyuka 7 | 4.274 | 9.392 |
| 15 | SPG4 | Mautu unity quatrter | 4.158 | 9.279 | 40 | BH5 | Slaughter house buea town | 4.288 | 9.403 |
| 16 | CAT1 | Mautu | 4.271 | 9.443 | 41 | BH6 | Great soppo | 4.231 | 9.339 |
| 17 | OW11 | Muea ,wonya meveo | 4.305 | 9.423 | 42 | BH7 | Bosta | 4.259 | 9.367 |
| 18 | OW12 | Yoke nangi street | 4.159 | 9.244 | 43 | SPG6 | Bafia | 4.365 | 9.314 |
| 19 | STM1 | Owe | 4.228 | 9.342 | 44 | OW24 | Malende 3 | 4.301 | 9.464 |
| 20 | STM2 | Ikata | 4.075 | 9.304 | 45 | SPR7 | Bonduma | 4.288 | 9.408 |
| 21 | OW13 | Matouke | 4.162 | 9.299 | 46 | BH8 | Bosta | 4.297 | 9.431 |
| 22 | OW14 | Koto 111 | 4.330 | 9.354 | 47 | OW25 | Yoke 3 | 4.302 | 9.779 |
| 23 | OW15 | Penda mboko | 4.298 | 9.381 | 48 | SPR8 | Bafia 2 | 4.319 | 9.431 |
| 24 | OW16 | Camp 1 | 4.309 | 9.463 | 49 | BH9 | Sandpit | 4.347 | 9.433 |
| 25 | BH1 | Bone quarter penda mboko | 4.299 | 9.463 | 50 | OW26 | Old police road muyuka | 4.343 | 9.431 |
Note: OW=Open Well, BH=Borehole, STM=Stream, CAT=Catchment, SPG=Spring.
Table 1: Details of sampling location.
Figure 2: Geologic map of the study area (modified from Che et al., 2012 and Wantim et al., 2013). Note:  Main road,
Main road,  Sedimentary cover.
Sedimentary cover.
Hydrogeology: The Mount Cameroon region is defined by its well-watered and frequently replenished heavy precipitation. The heavy rainfall that replenished the underground resources percolates through scoriaceous materials until it reaches the elevated water table. The region is characterized by an extensive network of springs and streams, hilly terrain and high humidity. Because of their abundance and connectivity, these faults can contain large amounts of running water in underground channels that flow in the form of large springs down the slope [15,25,26].
Because the pyroclastic component of Cameroonian acid is porous, it functions well as a large conservatory aquifer [15]. According to hydrogeology, the area is composed of several layers of massive basalt rocks broken by volcanic activity and likely to contain water, which is mainly found in these faults.
Three main water service providers operate in this region are Camerounaise Des Eaux (CDE), Community Water Schemes (CWS), and Private Boreholes (BH).
Gathering information for the field mapping came from outcrops near road cuts, wells, stream channels, springs, and river banks. The information is used as a source when making geological maps. Based on the guidelines for identifying aquifers and aquitards provided by Freeze et al. [27], lithology describes the physical makeup of a rock, including the mineral composition, grain size, and texture.
Physicochemical parameters (such as pH, temperature, EC, and TDS) were measured in-situ while samples of water are being collected. Due to their instability, the physico-chemical parameters were measured in-situ to prevent unexpected changes in characteristics and in accordance with accepted practices.
EC, pH, temperature and Total Dissolved Solids (TDS) were measured by a multimeter. The manufacturers' recommended buffer solution was used to calibrate these meters both before and during fieldwork. By plotting physicochemical data in the Piper-trilinear diagram, Wilcox diagram, and Gibbs diagram [5,28,29], the dominant hydrogeochemical facies and quality controlling mechanism of the study area were assessed. Statistical analyses were used to determine the maximum, minimum, mean, and correlation of physicochemical parameters [1,29]. Water quality parameters in the study area were compared to WHOrecommended standard guide values [30,31].
In order to find out the relationship between two chemical parameters, this study used a two-dimensional method using Correlation Matrix Analysis (CMA).
Spatial maps were created to estimate the groundwater quality in the study area.
Groundwater characterization and assessment using hydrogeochemistry
Table 2 lists the outcomes of the physico-chemical examination of the groundwater in the study area. It provides maximum, minimum and means values to illustrate the physical, major cation, and anion parameters 9 (Figure 3a).
| Elements | Min | Max | Mean |
|---|---|---|---|
| Ca2+ | 1.61 | 43.10 | 13.41 |
| Mg2+ | 0.00 | 20.20 | 8.71 |
| Na+ | 0.23 | 6.44 | 2.39 |
| K+ | 0.37 | 15.20 | 4.70 |
| NH4 | 0.00 | 18.30 | 0.93 |
| HCO-3 | 0.00 | 147.00 | 16.99 |
| CL- | 0.00 | 32.60 | 10.05 |
| SO2-4 | 0.00 | 20.13 | 4.54 |
| NO3 | 0.00 | 12.90 | 5.07 |
| TH | 13.69 | 152.51 | 69.89 |
| CA1 | 0.00 | 18.7 | 1.45 |
| CA11 | 0.00 | 18.7 | 1.45 |
| TDS | 11.00 | 211.00 | 102.24 |
| Temp | 23.10 | 32.10 | 27.69 |
| pH | 6.22 | 6.68 | 6.49 |
| EC | 22.00 | 425.00 | 204.88 |
Table 2: Basic statistics of the physicochemical parameters of groundwater.
Hydrogen's potential (pH)
The value represents the amount of hydrogen ions present in the groundwater. Every single groundwater sample (Figure 3b). Arithmetic mean pH value is 6.58, with pH values ranging from 6.49 to 6.68. Every sample fell within the WHO-recommended acceptable range.
Electrical Conductivity (EC)
A material's ability to conduct an electric current is measured by its Electrical Conductivity (EC), and a higher EC value indicates that salts have accumulated in the groundwater. The recommended EC limit value for drinking water is 1500 s/cm. With an arithmetic mean of 314.94 s/cm, the EC values in Figure 3c range from 205 to 425 s/cm.
Total Dissolved Solids (TDS)
Total dissolves solids in the study area were spatially distributed (Figure 3d) are 211 mg/l at the maximum and 102 mg/l at the minimum. It is safe to drink the water at every groundwater sampling site. According to WHO recommendations, water with TDS less than 600 mg/l is suitable for drinking with lower values in the sedimentary aquifer.
Chloride ions
Chloride's spatial distribution (Arithmetic mean value for (Figure 3e)) is 10.05 mg/l, with a minimum value of 0 mg/l and a maximum value of 32.6 mg/l. All samples in the investigation area are within the limit and suitable for drinking.
Bicarbonate ion
Mapping of spatial dispersion of bicarbonate ion is shown in Figure 3f. With an arithmetic mean of 16.99 mg/l, the values for bicarbonate ranged from 0 mg/l to 147 mg/l. Every water sample was within the limit and was suitable for drinking.
Nitrate ion
The most pervasive pollutants in subterranean environments are nitrogen compounds, which are primarily produced by nonpoint agricultural sources. Therefore, the public drinking water supply and public health are seriously threatened by an increase in nitrogen pollution. With a mean of 5.07 mg/l, the nitrate concentration ranged from 0 to 12.9 mg/l (Figure 3g). The permissible limit of 45 mg/l is not exceeded in any additional representative water samples.
Sulfate ions
The hydrochemical parameter becomes more dominant and is given higher weights as a result of the potential for sulfate pollution in groundwater to affect human health and cause material damage. Distribution of sulfates in space (0 to 20.13 mg/l, with an average of 4.54 mg/l, of (Figure 3h)) in groundwater was sampled. According to international and national standards, every water sample is within the allowable limits.
Magnesium and calcium ions
As shown in Figures 3i and l maps the spatial distribution of calcium and magnesium. Water hardness and abundant elements in surface and ground water are directly related to Ca2+ and Mg2+. Ca2+ concentration ranged from 1.61 to 43.1 mg/l, while Mg2+ concentration ranged from 0 to 20.2 mg/l. Magnesium is an abundant element that washes from rocks and is an alkali earth metal [32]. Magnesium is also used as a fertilizer for agricultural products and for a number of industrial applications (Figures 3j-3l).
Figure 3: Spatial dispersal of the physico-chemical parameters in the study area: (a) Temperature; (b) pH; (c) EC; (d) TDS; (e)Chlorides; (f) Bicarbonates; (g) Nitrate; (h) Sulfate; (i) Calcium; (j) Potassium; (k) Sodium and (l) Magnesium.
Total hardness
Different dissolved metallic ions, primarily in the form of Mg2+ and Ca2+ cations, contribute to water hardness. A minimum value of 13.69 mg/l CaCO3 and a maximum value of 152.51 mg/l CaCO3 are used to determine the TH content. All of the samples' calcium and magnesium concentrations are within acceptable limits (Figure 4) with the sedimentary aquifer showing higher values. This demonstrates that groundwater is harder in the form of Mg2+ than Ca2+.
Figure 4: Spatial distribution of the total hardness in the study area.
Sodium and potassium ions
Sodium concentration varied from 0.23 to 6.44 mg/l, and all of the water sampling points are within the permissible range. Sodium is the dominant ion among the cations and occurs in most of the natural waters. Sodium contributes about 53 to 69% of the total cations, this is primarily due to silicate weathering and dissolution of soil salts stored by the influence of evaporation, human activities, agricultural activities and poor drainage conditions. Potassium is a naturally occurring element, but its concentration remains lower than Ca, Mg and Na. The maximum value is found to be 15.2 mg/l and all the representative sampling points are within the permissible limit, indicating potassium complexes under the conditions investigated.
The relationship between hydrochemical parameters has been described using the Pearson rank coefficient correlation matrix presented in Table 3. It illustrates a significant link between EC and TDS (1:1), which reflects that EC is a function of TDS in groundwater [33]. Likewise, EC and TDS are insignificantly correlated with major cations such as Ca2+, Na+, K+, Mg2+ and major anions such as HCO−, SO2 −4, NO3 and Cl−, which indicates that TDS is not derived from these ions [11].
| Elements | Ca2+ | Mg2+ | Na+ | K+ | HCO3 | CL- | SO2-4 | NO3- | SiO2 | TDS | T | PH | EC | TH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | |||||||||||||
| Mg2+ | 0.12 | 1.00 | ||||||||||||
| Na+ | 0.46 | 0.10 | 1.00 | |||||||||||
| K+ | 0.85 | 0.14 | 0.48 | 1.00 | ||||||||||
| HCO3 | -0.10 | 0.69 | 0.00 | -0.06 | 1.00 | |||||||||
| CL- | 0.87 | 0.12 | 0.42 | 0.76 | -0.04 | 1.00 | ||||||||
| SO2-4 | 0.34 | 0.19 | 0.21 | 0.32 | 0.19 | 0.30 | 1.00 | |||||||
| NO3- | 0.54 | 0.01 | 0.33 | 0.35 | -0.14 | 0.58 | -0.21 | 1.00 | ||||||
| SiO2 | 0.18 | 0.01 | 0.34 | 0.20 | -0.04 | 0.11 | 0.44 | -0.04 | 1.00 | |||||
| TDS | 0.09 | -0.03 | 0.02 | 0.06 | -0.05 | 0.08 | -0.26 | 0.36 | -0.14 | 1.00 | ||||
| T | -0.09 | 0.08 | 0.00 | -0.10 | 0.18 | -0.11 | 0.26 | -0.37 | 0.03 | -0.62 | 1.00 | |||
| PH | 0.02 | 0.20 | -0.08 | 0.11 | 0.11 | -0.10 | -0.10 | 0.14 | 0.07 | 0.50 | -0.60 | 1.00 | ||
| EC | 0.09 | -0.03 | 0.02 | 0.05 | -0.05 | 0.08 | -0.25 | 0.35 | -0.14 | 1.00 | -0.62 | 0.50 | 1.00 | |
| TH | 0.87 | 0.59 | 0.43 | 0.76 | 0.26 | 0.77 | 0.37 | 0.44 | 0.15 | 0.06 | -0.03 | 0.11 | 0.06 | 1.00 |
Note: 
Table 3: Groundwater physicochemical parameter correlation matrix of the study area.
TH indicates a positive correlation of more than 0.8 and significantly influences the groundwater quality than other parameters. TDS; TH, Ca, Mg vs. Cl, and Ca vs. TH indicate a positive correlation of more than 0.87. The hardness present in the groundwater is in the form of CaCl2, MgCl2, and NaCl. The bicarbonate in groundwater shows a negative correlation with calcium and chloride.
The assessment of the correlation established by the binary graphs between the concentrations of major elements (SO42-, Mg2+, CL−, Ca2+, Na+, HCO3-) made it possible to identify the various mechanisms and processes that contributed to the mineralization of the sample waters. The Ca2+, Mg2+, HCO3- ions are formed in water during processes like dissolution, precipitation, dolomitization of carbonate rocks [28].
The contents of Ca2+ and Mg2+ have very variable concentrations, since these ions are involved in various processes of dissolution/ precipitation of gypsum, calcite and dolomite and again the phenomenon of base-exchange. The increase in Ca2+ levels that accompanied the low Mg2+ levels during the low water level period is due to the Base Exchange phenomenon, because the substratum clays can release Ca2+ ions after having fixed the Na+. This indicates that the origin of Ca2+ is not only the dissolution of calcite and gypsum, and thus confirms the hypothesis of a contribution of Ca2+ by ion exchange.
The HCO3- ion is weakly correlated with Ca2+ and Mg2+ meaning that calcium and magnesium evolve independently of bicarbonate, indicating that the dissolution of carbonate rocks (calcite, dolomite) is not the only source for these elements, because they also come from the dissolution of evaporates. This excess could be explained by the existence of another origin for Cl− ions (other than halides). This is related to another origin for this other than the dissolution of the halide. It may also have an anthropogenic origin (waste water).
Equation (1) is used to calculate the drinking water quality in the research area and determine the quality rating (Qi) for each component.
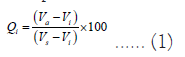
Where,
Qi=Quality ranking scale of element form a total number of water quality elements,
Va=actual groundwater quality concentration in the research location,
Vi=ideal rate of the water quality element can be realized from the standard tables.
Vi for pH=7, and other parameters, it equals zero.
Vs=standard for each chemical parameter.
The total DWQI was determined using Equation (2).
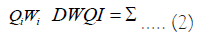
Table 4 provides classification information for groundwater quality based on index range. 29 samples (or 58%) were found to have excellent water quality (Rank I). While 13 (or 26% of the samples) exhibit good water quality for drinking (Rank II). The samples that fall into the fair, poor, and unfit categories, respectively, are 3 (6%) (Rank III), 4 (8%) (Rank IV), and 2 (4%) (Rank VI) (Figure 5).
| Range | Type of water | Sample Numbers |
|---|---|---|
| 0-25 | Excellent | 8,9,10,11,12,13,16,17,18,19,20,21,22,23,24,25,27,29,30,32,33,36,38,39,41,42,43,44,45,47,49,50 |
| 26-50 | Good | 1,5,7,14,26,28,31.34,37,46 |
| 51-75 | Fair | 2,3,4,15 |
| 76-100 | Poor | 6 |
| 101-150 | Very poor | |
| >150 | Unfit for drinking | 35,48 |
Table 4: Overall groundwater quality classification criteria based on GWQI.
Figure 5: Drinking water quality index in the study area.
Hydrogeochemical facies
To understand hydrochemical phenomena, key ion-chemical data is presented in three-linear Piper diagram. The relationship and behavior of the main anions and cations are explained by the hydrochemical facies of groundwater. Two triangles and a field in the shape of a rhombus make up the diagrams. As shown in Figure 6 central 3-rhombus-shaped shape field serves as a representation of the general hydrochemical properties of water samples.
Figure 6: Piper Trilinear Diagram showing the major cation and anions of groundwater samples in the study area. Note:  OW2, (
OW2, ( ) OW3,
) OW3, OW11,
OW11,  OW18.
OW18.
The concentration of the major anions (Cl−, SO4 2- and HCO) and cations (Ca2+, Mg2+, Na+, and K) in mg/l plotted in a Pipertrilinear diagram (Figure 6). Ca-Cl type (74%), Ca-HCO3 type (20%), and mixed Ca-Mg-Cl (6%) are the three different water types that are used to represent the geochemical evolution mechanism. The Mio-Plio Quaternary alluvial formations and gypsiferous marls dissolving together with the phenomenon of base-exchange, where Calcium ions are released by the clay minerals of the alluvial aquifers against adsorbed Na ions, can be used to explain the important and most dominant chloride calcium facies.
The piper diagram's cation-anion relationship demonstrates that calcium and chloride are highly concentrated in the study area as a result of weathering, silicate dissolution in soils or rock salts through evaporation, and human-induced activities. According to the Piper trilinear diagram, the study area has high concentrations of calcium and chloride as a result of weathering and the silicate in rock salts or soil dissolving due to evaporation and human-caused activities.
Mechanism of groundwater
The Gibbs plot [34] is frequently used to comprehend the mechanism controlling the cation and anion chemistry of groundwater. According to three zones--precipitation dominance, vaporization dominance, and rock dominance--the plot is divided (Figure 7). TDS values were plotted against cation ratio (Na+K)/ (Na+K+Ca) and anion ratio (Cl)/(Cl−+HCO−3) as shown in Figures 3a and 3b. The majority of groundwater samples (76%) were found in the rock dominance zone, whereas only 14% were in the precipitation dominance zone, suggesting that the interaction between rocks and water is the primary controlling factor in groundwater chemistry Gibb's plot reveals that the chemical weathering of the rock forms minerals. The Gibbs ratio I (cation) value in the study area varied from 0.11 to 0.96 with a mean value of 0.38, and the Gibbs ratio II (anion) values ranged from 0.11 to 0.97 with a mean value of 0.66. Geogenic and anthropological induced activity and urbanization have increased the TDS value and tended the samples to fall into the rock and precipitation dominance zone. Ion exchange reactions between water and rocks were assessed by calculating the Chloro-Alkaline Indices (CAI). Two Chloro-Alkaline Indices CAI 1 and CAI 2 is (were) used for the interpretation of ion exchange between groundwater and host environment. Equation 3 and 4 express the CAI with concentration in meq/L.
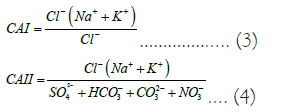
Figure 7: Mechanism controlling the chemistry of groundwater. Note:  TDS values.
TDS values.
A positive Chloro-Alkaline Indices indicates exchange of Na+ and K+ from the water with Mg2+ and Ca2+ of the water with Na+ and K+ of the rocks (base reaction). The CAI is negative when there is an exchange of Mg2+ and Ca2+ of the water with Na+ and K+ of the rocks. All of the samples have positive CAI which indicates the reverse process that is sodium and potassium in the waters substituting Mg2+ and Ca2+ in the rocks [35], and positive results with the base-exchange reaction between Na+, K+, Mg2+ and Ca2+ in groundwater. High base-exchange reaction in which alkali earths (HCO-3>Mg2++ Ca2+) may be referred to as base-exchange softened water, whereas Na+ ion is exchanged for alkali earths (Mg2++ Ca2+> HCO-3) can be referred to as base-exchange hard water [36].
Irrigation water quality indices
Assessment of irrigation water quality indices: The mineral content of the soil and water has an impact on the suitability of irrigation water as well [37]. Depending on how mineral water elements affect the plants and soil, groundwater may or may not be suitable for irrigation [38,39]. A reasonable yield will not be achieved with good quality water in an area with poor drainage. The following methods are used to calculate the total salt concentration: Electrical conductivity, Sodium Adsorption Ratio (SAR), Kelly Ratio (KR), Soluble Sodium Percentage (SSP), Permeability Index (PI), and Magnesium Adsorption Ratio (MAR). The concentration of all ions is expressed as meq/l, and these parameters are used to assess the quality of irrigation water.
Sodium Adsorption Ratio (SAR): To determine whether ground water is suitable for irrigation, this indicator is crucial [39,40]. The equation in Equation (4) is used to calculate the alkali/ sodium threat to crops.
Sodium percent (Na%): The sodium content of irrigation waters is typically expressed as a percentage of sodium. To determine a natural water's suitability for irrigation, Wilcox, et al. [17] noted that Na percent is a common parameter (Figure 8). The following equation was used to calculate the sodium percent (Na percent) values:
Figure 8: Wilcox diagram showing the suitability of groundwater for irrigation in the study area. Note:  TDS values.
TDS values.
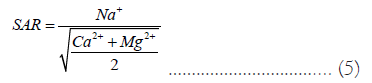
Where all ionic concentrations are expressed in mg/l. According to the Wilcox diagram comparing sodium percent and total concentration, and all of the samples fell in the good to permissible for irrigation category.
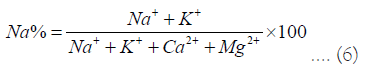
Kelly ratio: Based on the ratio of sodium against Ca and Mg ions, as shown in Equation (9), the Kelly ratio [41] is used to classify the quality of irrigation water (Table 5).
| Range | KR | No. of samples | % of sample |
|---|---|---|---|
| <1 | Suitable | 50 | 100 |
| 1–2 | Marginal | NIL | – |
| >2 | Unsuitable | NIL |
Table 5: Classification of groundwater based on KR.
Soluble Sodium Percentage (SSP): SSP was determined using the measured concentration of Na+, K+, Ca2+, and Mg2+, expressed in meq l-1 shown in Eq. 7. SSP values ranges from 22.24 to 370.10 shown in Table 6.
| Range | Category | Categories | Percentage |
|---|---|---|---|
| <20 | Excellent | 49 | 98 |
| 20–40 | Good | 1 | 2 |
| 40–80 | Fair | Nil | Nil |
| >80 | Poor | Nil | Nil |
Table 6: Classification of groundwater based on SSP.
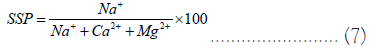
Magnesium Hazard (MH): In general, Ca2+ and Mg2+ maintain a state of equilibrium in most waters). In addition, high concentrations of Ca2+ and Mg2+ in groundwater can degrade soil quality, which adversely affects the crop yield [42]. In order to determine whether water is suitable for irrigation, the Magnesium Hazard (MH) ratio is very helpful [43]. A MH ratio of more than 50% is regarded as being unsuitable for irrigation. It is computed utilizing Eq. 8 .
MH ratio ranged from 21.51 to 45.09% which is less than 50% in all the samples indicating good quality of water for irrigation.
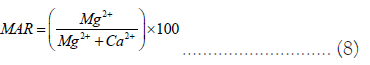
Permeability Index (PI)
It's important to analyze irrigation water quality in relation to soil permeability that is impacted by Na, Mg, Ca, and HCO3. Equation (9) evaluates the permeability index to determine the water mobility in the soil [4,44].
Plant growth and soil permeability are directly related; low permeability soils are insufficient for plant growth. Classify the groundwater quality in relation to the Permeability Index (PI) and the total ion concentration (in meq/L). The permeability index, or unit of soil permeability, is defined in equation (Eq. 9, where all ions are measured in meq/L. There are three classes of permeability index: class I, which has permeability at 100% of its maximum, class II, which has permeability at 75% of its maximum, and class III, which has permeability at 25% of its maximum. Class I indicates suitability for irrigation, Class II indicates marginal suitability, and Class III indicates unsuitability. According to PI, 6% of the groundwater samples are suitable (class I), 44% of the samples are marginally suitable (class II) for irrigation, and 50% of the samples are unsuitable (class III).
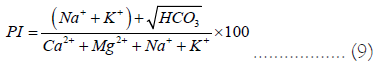
Residual sodium carbonate
The US Department of Agriculture states that water containing more than 2.5 meq/l of RSC is not suitable for irrigation. Utilizing the formula suggested by Eaton, et al. [45] and Fitton, et al. [46] the RSC value of surface water samples was calculated. The range of the RSC values for the water sample was between -3.05 and 0.24 meg/l. Water is suitable for irrigation purposes as shown by 100% of samples having values less than 1.25 meq/l (Tables 7 and 8) [47-59].
| Range | Category | Categories | Percentage |
|---|---|---|---|
| <1.5 | Excellent | 50 | 100 |
| 1.5-3.0 | Good | Nil | Nil |
| 3.0-6.0 | Poor | Nil | Nil |
| >6.0 | Unsuitable | Nil | Nil |
Table 7: Classification of groundwater based on RSC.
| Sr. No. | TH | %SH | RSC | SAR | PI | MAR | KR | SC | Na% | SSP | EC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 108.54 | 10.43 | 0.24 | 0.11 | 73.07 | 71.70 | 0.06 | 4.82 | 9.91 | 5.23 | 212.00 |
| 2 | 118.06 | 6.65 | -1.19 | 0.09 | 47.96 | 63.82 | 0.04 | 3.68 | 6.38 | 4.10 | 282.00 |
| 3 | 90.22 | 12.26 | -0.10 | 0.11 | 73.95 | 86.27 | 0.06 | 3.74 | 11.62 | 5.58 | 64.00 |
| 4 | 37.59 | 9.83 | -0.71 | 0.09 | 30.68 | 80.07 | 0.08 | 0.86 | 9.16 | 7.00 | 58.00 |
| 5 | 51.60 | 7.46 | -0.83 | 0.04 | 45.28 | 69.99 | 0.03 | 1.31 | 7.25 | 3.03 | 25.00 |
| 6 | 48.17 | 13.97 | -0.76 | 0.13 | 50.97 | 70.86 | 0.10 | 1.30 | 12.81 | 8.71 | 22.00 |
| 7 | 142.47 | 13.43 | -2.84 | 0.06 | 4.34 | 29.18 | 0.02 | 3.28 | 13.15 | 2.33 | 41.00 |
| 8 | 47.21 | 31.93 | -0.94 | 0.41 | 22.90 | 54.24 | 0.30 | 1.25 | 24.80 | 22.90 | 61.00 |
| 9 | 41.56 | 5.82 | -0.82 | 0.05 | 14.18 | 78.36 | 0.04 | 0.89 | 5.63 | 3.44 | 111.00 |
| 10 | 116.90 | 12.75 | -2.34 | 0.01 | 0.43 | 36.98 | 0.00 | 2.68 | 12.70 | 0.43 | 271.00 |
| 11 | 89.73 | 18.74 | -1.76 | 0.20 | 18.13 | 38.77 | 0.11 | 2.19 | 17.09 | 9.59 | 289.00 |
| 12 | 33.70 | 30.23 | -0.61 | 0.21 | 46.00 | 66.58 | 0.18 | 0.97 | 26.25 | 15.13 | 80.00 |
| 13 | 104.77 | 1.88 | -2.09 | 0.01 | 0.48 | 39.69 | 0.00 | 2.13 | 1.87 | 0.48 | 266.00 |
| 14 | 63.81 | 23.87 | -1.28 | 0.20 | 11.15 | 17.03 | 0.13 | 1.62 | 21.52 | 11.15 | 266.00 |
| 15 | 78.44 | 16.65 | -1.57 | 0.21 | 10.40 | 65.61 | 0.12 | 1.84 | 15.01 | 10.40 | 269.00 |
| 16 | 85.49 | 13.83 | -1.67 | 0.09 | 14.83 | 31.06 | 0.05 | 2.00 | 13.28 | 4.43 | 269.00 |
| 17 | 46.02 | 22.08 | -0.78 | 0.25 | 49.71 | 66.30 | 0.18 | 1.27 | 18.79 | 15.51 | 244.00 |
| 18 | 38.08 | 12.36 | -0.25 | 0.09 | 94.12 | 59.14 | 0.07 | 1.37 | 11.59 | 6.57 | 342.00 |
| 19 | 62.45 | 13.54 | -1.25 | 0.08 | 4.65 | 46.01 | 0.05 | 1.43 | 12.97 | 4.65 | 240.00 |
| 20 | 35.04 | 24.23 | -0.68 | 0.13 | 28.38 | 64.51 | 0.11 | 0.92 | 22.10 | 10.16 | 143.00 |
| 21 | 68.56 | 8.64 | -0.50 | 0.06 | 69.15 | 60.66 | 0.04 | 2.36 | 8.35 | 3.46 | 176.00 |
| 22 | 39.01 | 13.83 | -0.48 | 0.13 | 73.38 | 57.74 | 0.10 | 1.19 | 12.60 | 9.27 | 94.00 |
| 23 | 58.41 | 11.79 | -1.14 | 0.05 | 16.36 | 36.72 | 0.04 | 1.34 | 11.43 | 3.38 | 81.00 |
| 24 | 71.22 | 14.79 | -1.28 | 0.12 | 31.12 | 37.23 | 0.07 | 1.79 | 13.89 | 6.62 | 280.00 |
| 25 | 121.07 | 15.53 | -1.81 | 0.14 | 36.24 | 32.13 | 0.06 | 3.45 | 14.71 | 5.79 | 58.00 |
| 26 | 61.13 | 25.82 | -1.20 | 0.32 | 26.54 | 39.13 | 0.20 | 1.58 | 21.70 | 16.92 | 293.00 |
| 27 | 47.67 | 13.18 | -0.82 | 0.13 | 44.18 | 91.56 | 0.10 | 1.22 | 12.06 | 8.83 | 185.00 |
| 28 | 40.27 | 17.73 | -0.77 | 0.21 | 34.15 | 81.08 | 0.17 | 0.98 | 15.21 | 14.36 | 57.00 |
| 29 | 37.39 | 10.29 | -0.22 | 0.08 | 97.28 | 58.58 | 0.07 | 1.35 | 9.67 | 6.28 | 187.00 |
| 30 | 77.93 | 10.14 | -1.56 | 0.10 | 5.24 | 67.63 | 0.06 | 1.72 | 9.63 | 5.24 | 209.00 |
| 31 | 96.07 | 11.70 | -0.19 | 0.11 | 70.16 | 86.58 | 0.06 | 3.88 | 11.10 | 5.42 | 305.00 |
| 32 | 85.12 | 19.46 | -1.66 | 0.18 | 19.93 | 31.05 | 0.10 | 2.11 | 17.86 | 9.09 | 273.00 |
| 33 | 116.40 | 19.51 | -2.15 | 0.19 | 24.59 | 37.14 | 0.09 | 3.02 | 18.13 | 7.94 | 154.00 |
| 34 | 38.74 | 2.93 | -0.74 | 0.02 | 24.37 | 77.37 | 0.01 | 0.83 | 2.89 | 1.28 | 242.00 |
| 35 | 27.95 | 12.84 | -0.56 | 0.05 | 13.27 | 82.49 | 0.05 | 0.64 | 12.27 | 4.82 | 248.00 |
| 36 | 51.17 | 11.33 | -0.62 | 0.11 | 64.76 | 58.17 | 0.08 | 1.54 | 10.53 | 7.30 | 161.00 |
| 37 | 152.51 | 19.46 | -3.05 | 0.23 | 8.42 | 29.42 | 0.09 | 3.72 | 18.00 | 8.42 | 219.00 |
| 38 | 83.84 | 26.90 | -1.63 | 0.22 | 21.89 | 48.47 | 0.12 | 2.26 | 24.47 | 10.67 | 219.00 |
| 39 | 43.13 | 3.50 | -0.86 | 0.02 | 1.25 | 79.15 | 0.01 | 0.89 | 3.45 | 1.25 | 219.00 |
| 40 | 116.81 | 19.52 | -2.33 | 0.18 | 7.53 | 37.36 | 0.08 | 2.85 | 18.22 | 7.53 | 239.00 |
| 41 | 79.59 | 10.20 | -1.59 | 0.09 | 4.82 | 66.74 | 0.05 | 1.76 | 9.74 | 4.82 | 280.00 |
| 42 | 85.74 | 18.37 | -1.67 | 0.18 | 19.80 | 31.26 | 0.10 | 2.10 | 16.87 | 8.91 | 342.00 |
| 43 | 45.92 | 10.32 | -0.76 | 0.10 | 47.48 | 65.63 | 0.07 | 1.18 | 9.63 | 6.89 | 425.00 |
| 44 | 38.16 | 10.60 | -0.25 | 0.08 | 94.62 | 59.02 | 0.07 | 1.36 | 9.95 | 6.36 | 209.00 |
| 45 | 64.68 | 14.45 | -1.29 | 0.10 | 5.77 | 47.87 | 0.06 | 1.50 | 13.69 | 5.77 | 299.00 |
| 46 | 34.41 | 11.28 | -0.67 | 0.09 | 26.63 | 66.54 | 0.08 | 0.79 | 10.48 | 7.33 | 235.00 |
| 47 | 69.37 | 10.59 | -0.41 | 0.10 | 72.69 | 62.92 | 0.06 | 2.51 | 10.05 | 5.40 | 166.00 |
| 48 | 13.68 | 50.71 | -0.27 | 0.15 | 16.81 | 0.00 | 0.20 | 0.50 | 45.09 | 16.81 | 328.00 |
| 49 | 119.16 | 19.43 | -1.77 | 0.23 | 39.15 | 31.89 | 0.11 | 3.50 | 17.75 | 9.52 | 234.00 |
| 50 | 43.15 | 3.42 | -0.86 | 0.02 | 1.15 | 79.10 | 0.01 | 0.89 | 3.39 | 1.15 | 272.00 |
Table 8: Values of irrigation indices for assessing irrigation water quality.
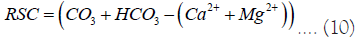
Groundwater samples' hydrochemical analyses revealed that the water is mildly acidic, moderately mineralized, and soft to moderately hard. Groundwater had temperature readings between 23 and 32ºC. Ca>Mg >K>Na=HCO3>Cl>SO4>NO3 is the order of the abundance of the major cations and anions. Groundwater in the study area is primarily of the Ca-Cl type (74%) followed by Ca-HCO3 type (20%) and mixed Ca-Mg-Cl (6%) hydrochemical facies. The study area's high levels of EC, calcium, magnesium, sodium, chloride, and sodium showed that base-exchange and the mechanism involving rock-water interaction are the main causes of the deterioration of the water quality.
Hydrogeochemical investigations, WHO and BIS standards, the groundwater samples can be used for drinking. Most groundwater sources fall into the excellent to good category, which denotes medium salinity, low sodium water that can be used to irrigate all types of soil without running the risk of exchangeable sodium. Bicarbonate is the predominant anion, and calcium is the main cation, according to the data. The spatial dispersal mapping of GWQI values at the unobserved locations of the study area can also be utilized to ameliorate groundwater quality.
On examination of suitability of water towards agricultural use, it was found that, based on the RSC classification, 100% of the samples fell in the excellent category. According to SSP analysis, 98 percent of the samples fell into the excellent category, and 2% of the samples fell into the good category for irrigation. All of the samples from the SAR versus EC analysis met the requirements for irrigation, as shown by the Wilcox diagram. The current study will support long-term preventive action by competent authorities and policymakers.
We would like to thank Prince Tanyi Justin Aginga of Union Farms of Africa, for providing assistance in the handling of the samples in India, correlating with the laboratory technicians for timely analyses of Samples.
The authors declare no conflict of interest
Mr. Ngai N Jude carried out the field work, conceived the idea, and wrote the draft manuscript. Prof. Agyingi, Dr. Engome Regina Wotany and Dr. Franck Eitel Kemgang Ghomsi. Proof read the manuscript and made suggestions. Maffo Kankeu Gisele Aurore, Menti Agbor Nelson and, Armel Zacharie Ekoa Bessa made useful corrections and read the manuscript.
The data generated and analyzed during the study are available from the corresponding author on reasonable request.
Citation: Jude NN, Wotany ER, Agyingi C, Ghomsi FEK, Aurore MKG, Nelson MA, et al (2023) Hydrogeochemical Characterization of Groundwater Quality, East of Mount Cameroon and West of the Penda Mboko River, Suitability for Drinking and Irrigation Use. J Geol Geophys. 12:1093.
Received: 12-Apr-2023, Manuscript No. JGG-23-23479; Editor assigned: 14-Apr-2023, Pre QC No. JGG-23-23479 (PQ); Reviewed: 28-Apr-2023, QC No. JGG-23-23479; Revised: 05-May-2023, Manuscript No. JGG-23-23479 (R); Published: 12-May-2023 , DOI: 10.35248/2381-8719.23.12.1093
Copyright: © 2023 Jude NN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.