
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Review - (2023)Volume 14, Issue 4
Primary Immuno Deficiency (PID) disorders are heterogeneous disorders of the innate or the adaptive immune system, leading to recurrent infections that can also predispose to autoimmune diseases and malignancies. Hyper IgM Syndromes (HIGM) are rare disorders characterized by defective Class Switch Recombination (CSR) and/or Somatic Hypermutation (SHM) resulting in decreased levels of IgG, IgE and IgA antibodies and normal or elevated IgM levels. Various genetic defects have been identified to cause HIGM syndromes. These include intrinsic B cell defects or defects in the interaction between T and B cells, leading to a clinical phenotype of either pure humoral immunodeficiency or combined immunodeficiency respectively. HIGM syndromes with combined immunodeficiency present with recurrent opportunistic infections, notably Pneumocystis jiroveci and Cryptosporidium parvum infections, neutropenia and autoimmune complications, whereas the milder HIGM syndromes with humoral immunodeficiency are characterized by recurrent sinopulmonary and gastrointestinal infections with encapsulated bacteria. Patients with clinical suspicion of HIGM syndromes should undergo measurement of serum immunoglobulin levels followed by flow cytometry for lymphocyte subset analysis. Genetic testing is required for the final confirmation of all HIGM syndromes. Early identification and timely referral to the clinical immunologist for management is vital for the prevention of significant patient morbidity. Therapeutic options include immunoglobulin replacement therapy for the humoral immunodeficiency variants and Hematopoietic Cell Transplantation (HCT) for the more severe CD40 Ligand (CD40L) deficiency. This review aims to provide a concise clinical approach to PID disorders in general, followed by an overview of the pathogenesis, clinical features and management of various HIGM syndromes.
Class switch recombination; Humoral immunodeficiency; Hyper IgM syndromes; Primary immunodeficiency disorders
Primary Immunodeficiency (PID) disorders also known as Inborn Errors of Immunity (IEI), are heterogeneous disorders characterized by defects in the immune system, leading to increased risk of infections, autoimmune diseases and malignancies [1,2]. Either the innate or the adaptive immune system or both may be defective and most of them are inherited disorders, but it is important to note that few acquired forms have also been recognized [3]. Starting form a meagre 13 PID disorders recognized 40 years ago to currently over 485 individual PID disorders having been identified, research into the field of PID has enlightened the medical fraternity of the immunological framework that controls host defence, autoimmunity and malignancy [4,5]. Over 6 million people worldwide suffer from PID, however 70%-90% of cases remain undiagnosed [6] owing primarily to the lack of awareness among primary care physicians and lack of advanced diagnostic facilities in Low and Middle Income Countries (LMIC) [7]. The prevalence of PID varies between 1 in 1000 to 1 in 5000 among different ethnicities [8]. Among the various PID disorders, it has been estimated that the most common disorder is Common Variable Immune-Deficiency (CVID) (35% of PID), followed by IgA deficiency (26% of PID) [9].
The International Union of Immunological Sciences (IUIS) has classified the various PID disorders into several broad categories, the simplified version of the same has been shown in Table 1 [10]. The clinical features of PID disorders are highly variable, albeit they usually present as routine infections of the sinuses, ears and lungs which are extremely susceptible to be missed in the primary care setting. Thus, early suspicion and prompt referral to the clinical immunologist when required is vital for timely identification and management of PID disorders before significant patient morbidity ensues (Table 1) [11,12].
| S. No | Category | Examples of IEI in the category |
|---|---|---|
| 1 | Immune-deficiency affecting cellular and humoral immunity | |
| a) | Severe combined immune-deficiency (SCID) (CD3 T cell < 300/µL) | γC def, JAK3 def, ADA def, RAG 1/ 2 def |
| b) | Combined immune-deficiency less profound than SCID | CD8 def, MHC I def, ICOS def, CARD 11 def, CD40L/CD40 def |
| 2 | Combined immune-deficiency with syndromic features | a) WAS (with thrombocytopenia) |
| b) DNA repair defects (AT, NBS, Bloom sd, PMS2 def) | ||
| c) Immuno-osseus dysplasia (Schimke sd) | ||
| d) Thymic defects (DiGeorge sd, CHARGE sd) | ||
| e) Hyper IgE syndromes | ||
| f) EDA-ID (NEMO def) | ||
| 3 | Predominantly antibody deficiencies | |
| a) | Hypogammaglobulinemia (↓IgG, IgA, IgM) | CVID phenotype, X-linked agammaglobulinemia, CD19 def |
| b) | Other antibody deficiencies (↓↓IgG,IgA with N/↑ IgM) | HIGM syndromes |
| 4 | Diseases of immune dysregulation | |
| a) | HLH and EBV susceptibility | Familial HLH, Chediak Higashi sd, Griscelli sd, XLP 1/2 |
| b) | Syndromes with autoimmunity | APECED, ALPS, IL 10 def, IPEX |
| 5 | Congenital defects of phagocytes | |
| a) | Neutropenia | Shwachman- Diamond sd, Barth sd |
| b) | Functional defects | CGD, LAD I/II, cystic fibrosis, PAP |
| 6 | Defects in intrinsic and innate immunity | |
| a) | Bacterial, fungal and parasitic infections | MyD88 def, IL 17 def, IL 17R def, CARD9 def, CMC sd |
| b) | MSMD and viral infections | Complete IFNGR1 def, WHIM sd |
| 7 | Auto inflammatory disorders | TRAPS, FMF, Mevalonate kinase def, NOMID, CAPS |
| 8 | Complement deficiencies | |
| a) | High susceptibility to infection | Recurrent Neisserial infections (C5, C6, C7, C8, C9 def) |
| b) | Low susceptibility to infection | SLE like syndromes (C1q, C1r, C1s, C2 def), Atypical HUS syndromes (Factor H def, Factor I def) |
| 9 | Bone marrow failure | Fanconi anemia, Dyskeratosis congenita |
| 10 | Phenocopies of PID | Cryopyrinopathy, With auto-antibodies (CMC, PAP, Good sd) |
Table 1: Simplified version of IUIS classification of human IEI.
B cell immune-deficiency disorders as a group comprise nearly 50% of all PID disorders and occur either due to intrinsic molecular defects in B cells or due to faulty interaction (‘cross-talk’) between B and T cells [11,13]. B cell immune-deficiency disorders are distinguished from other PID disorders primarily by the age of onset and clinical features. Increased susceptibility to infection with encapsulated bacteria (eg: Streptococcus pneumoniae, Haemophilus influenzae) is a hallmark of B cell immune-deficiencies whereas T cell/combined immune-deficiencies are characterized by opportunistic infections and severe viral infections [13].
Hyper Immunoglobulin M Syndromes (HIGM) are rare PID disorders characterized by defective Class Switch Recombination (CSR) [14] and/or Somatic Hypermutation (SHM) [4] resulting in decreased IgA, IgG and IgE and normal or increased IgM levels [15]. They can manifest either as combined immune-deficiency when the defect lies at the level of B cell-T cell interaction or as a pure humoral immune-deficiency when intrinsic B cell defects pertaining to CSR are present [16]. In this review, we aim to provide a concise clinical approach to PID disorders in general and HIGM syndromes in particular; especially catering to primary care physicians in resource limited settings.
Hyper immunoglobulin M syndromes
The discovery of HIGM syndromes had its roots in 1961 when Rosen et al., [17] described recurrent infections in two brothers and Burtin [18] reported a similar patient with low IgG levels and high IgM levels. Owing to this discrepancy between IgG and IgM levels, the term ‘dysgammaglobulinemia’ was used initially which was later termed ‘immunodeficiency with hyper IgM’ in 1974 by a World Health Organization working party [19]. In this review we aim to provide a brief overview of the various HIGM syndromes encountered in clinical practice (Table 2).
| S. No | HIGM syndrome | Mode of inheritance | Chromosome location and gene involved | Clinical features | Management |
|---|---|---|---|---|---|
| 1 | HIGM 1 (CD40L deficiency) (CID) | X linked | Xq26.3; CD40L gene | Recurrent respiratory infections; chronic diarrhoea; opportunistic infections with Pneumocystis jiroveci, Cryptosporidium parvum; sclerosing cholangitis; neutropenia | HCT; Ig replacement; Prophylaxis against opportunistic infections; management of neutropenia with GCSF; Gene therapy and rCD40L |
| 2 | HIGM 2 (AID deficiency) and HIGM 4(HID) | AR/AD | 12p13.31; AICDA gene | Sino-pulmonary infections and gastrointestinal infections; lymphadenopathy; autoimmunity; splenomegaly; giant germinal centres | Ig replacement therapy; immunosuppressive drugs for autoimmunity |
| 3 | HIGM 3 (CD40 deficiency) (CID) | AR | 20q13.12; CD40 gene | Recurrent opportunistic infections with Pneumocystis jiroveci, Cryptosporidium parvum; sclerosing cholangitis; neutropenia | HCT (less effective than for HIGM 1); Ig replacement; Prophylaxis against opportunistic infections; management of neutropenia with GCSF |
| 4 | HIGM 5 (UNG deficiency) (HID) | AR | 12q24.11; UNG gene | Recurrent sinopulmonary infections and lymphadenopathy | Ig replacement therapy |
| 5 | HIGM 6/ EDA-ID (NEMO defect) | X linked | Xq28; NEMO/ IKKγ gene | Recurrent respiratory infections; ectodermal dysplasia; dry scaly skin; conical teeth; Mycobacterium avium infections; osteopetrosis; absent eccrine sweat glands | Ig replacement; prophylaxis against opportunistic infections |
| 6 | APDS (PIK3CD defect) | AD | 1p36.22; PIK3CD gene | Recurrent sino-pulmonary infections; bronchiectasis; orbital cellulitis; severe CMV, EBV, HSV and VZV infections; lymphadenopathy; splenomegaly; lymphoproliferative disorders | Ig replacement; Leniolisib |
Table 2: Summary of important HIGM syndromes.
Pathogenesis of HIgM syndromes
HIGM syndromes are PID disorders characterized by defective CSR and/or SHM resulting in markedly reduced levels of IgG, IgA and IgE and normal/elevated IgM levels [20]. To understand the root cause of HIGM, it becomes important to have a basic understanding of CSR and SHM (Figure 1).
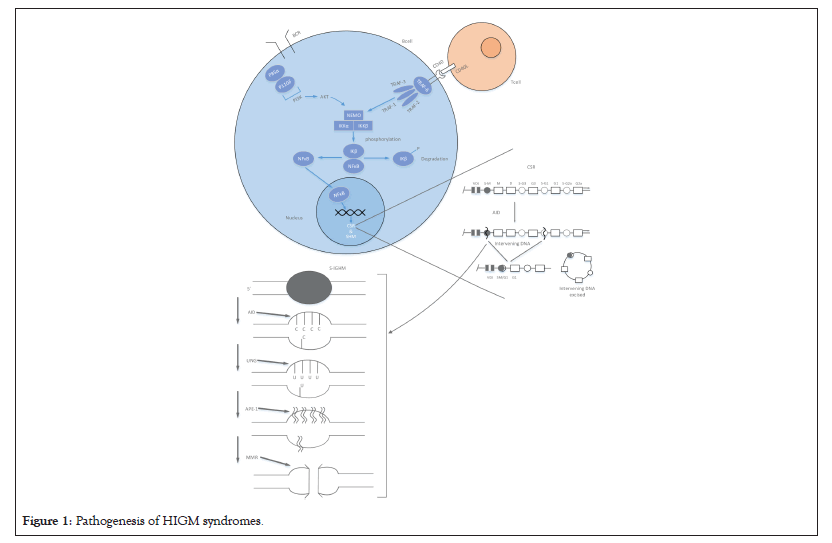
Figure 1: Pathogenesis of HIGM syndromes.
CSR is the process of relocation of the previously constructed unique V(D)J combination from its association with IGHM (constant region gene for IgM) to IGHG, IGHA or IGHE which are the constant region genes for IgG, IgA or IgE respectively [21]. CSR is a complex process which involves creation of dsDNA breaks, excision of the intervening sequences and dsDNA repair (Figure 1). Recombination occurs between switch regions that are found flanking each constant region gene at the 5’ end. CSR begins with DNA transcription at a point upstream from the S regions which creates ssDNA substrates for the enzyme Activation Induced Cytidine Deaminase (AID). AID deaminates cytidine into uracil residues [22] which are subsequently excised by the enzyme Uracil N Glycosylase (UNG) [23]. This is followed by the creation of single-strand breaks in the DNA strand by an endonuclease like Apurinic/Apyrimidinic Endonuclease 1 (APE-1). Mismatch Repair (MMR) complex of proteins (eg: PMS2) has been implicated in converting ssDNA breaks into dsDNA breaks [24], thus facilitating the excision of the intervening segment which is then followed by DNA repair [25]. SHM generates numerous mutations in IGHV (gene for the variable region) and B cells harbouring these mutated IGHV genes with higher antibody affinity are preferentially selected for germinal centre proliferation and thus achieve affinity maturation [26]. SHM is less understood than CSR, however it is known that AID function is central to SHM and dsDNA breaks occur in this process as well [27].
HIGM syndromes encompass disorders which are caused due to defective CD40L-CD40 interaction, Nuclear Factor Kappa-B (NFκB) signalling defects, DNA repair defects or deficiency of enzymes like AID or UNG [16].
CD40L-CD40 interaction is the first step in CSR and SHM as shown in Figure 1. X-linked recessive CD40L deficiency (HIGM 1) was the first HIGM syndrome to be recognized and is the most common amongst all HIGM syndromes, comprising nearly 70% of cases [28]. CD40L, a 39kDa glycoprotein (member of the tumour necrosis factor family) has three functional domains, namely the extracellular, transmembrane and intracellular domains [29]. Most of the CD40L gene mutations including single amino acid substitutions, truncations, in-frame or out-of-frame deletions, nonsense, missense, insertion and splice-site mutations affect the extracellular domain of CD40L resulting in defective geometric folding of CD40L [30]. Promoter region abnormalities of the CD40L gene have also been identified to cause X-HIGM [31]. In addition to defective CSR and SHM, lack of CD40L expression also results in defective T cell interaction with macrophages affecting the production of T-Helper Cell Type 1 (Th1) cytokines (interleukin 12, interferon-gamma) [32] resulting in increased susceptibility to opportunistic intracellular pathogens like Pneumocystis jiroveci [33], Cryptosporidium species [34], Toxoplasma gondii [35] and Mycobacteria species [36].
CD40 (member of the TNF receptor family), expressed on antigen presenting cells (B cells, macrophages and dendritic cells) is defective in autosomal recessive HIGM [37] and is highly under-reported compared to other forms of HIGM [38]. In the resting state NFκB dimers are sequestered in the cytoplasm through interaction with inhibitory proteins like IK-B proteins (Figure 1). NEMO/IKKγ serves as a scaffold for two other proteins namely IKKα and IKKβ. Cytokine activation of the cell results in activation of IKKα and IKKβ which results in hyperphosphorylation of IK-B proteins which ultimately results in ubiquitin mediated proteasomal degradation of IK-B proteins. This frees NFκB from IK-B proteins and allows the nuclear translocation of NFκB [39]. NFκB signalling defects also result in rare forms of HIGM syndromes namely, X-linked anhidrotic Ectodermal Dysplasia with Immune-Deficiency (EDA-ID) due to a mutation in the NFκB essential modulator (NEMO/IKBKG) [40] gene and another clinically overlapping syndrome with autosomal dominant inheritance caused by mutations in the NFKB1A gene [41].
AID and UNG enzymes are central to the process of CSR and SHM as mentioned earlier. AID is transiently expressed in germinal centre B cells following activation of CD40 [16]. Homozygous mutations including amino acid substitutions, premature stop codons and deletions, particularly in exon 3 of the AICDA gene result in autosomal recessive HIGM (HIGM 2) [42,43]. Recently heterozygous mutations in the carboxy terminal end of AICDA gene have been reported to cause autosomal dominant HIGM which is distinct from HIGM 2 by having intact SHM [44]. Mutations in the gene encoding UNG (all affecting the glycosylase domain) have also been recognized to cause HIGM [45]. Both AID and UNG deficiencies are pure B cell immune-deficiency disorders and are characterized by normal numbers of CD19+ B cells and CD27+ memory B cells [20].
CD40-CD40L interaction leads to activation of PI3K enzymes which in turn leads to production of reactive oxygen species and modulation of CD40 expression [46]. Heterozygous gain of function mutations in the p110-δ catalytic sub-unit and the p85-α regulatory subunit have been recently found to cause Activated PI3K Delta Syndrome (APDS) and SHORT syndrome respectively, both being associated with the HIGM phenotype [30,47].
PMS2, MSH2, MSH6 and INO80 genes encode proteins which are components of the mismatch repair machinery involved in CSR-induced generation of ds-DNA breaks [48,49]. Proteins encoded by the MRE11 and NBN genes are pivotal for the detection and recruitment of DNA repair machinery during CSR [50]. Mutations in these DNA repair genes have also been associated with the HIGM phenotype, as have syndromes like Nijmegen breakage syndrome and Ataxia Telangiectasia [50].
Clinical manifestations of hyper IgM syndromes
The clinical presentations of PID disorders are highly variable. Technical advancements in the form of whole-genome and RNA sequencing have progressed much faster than can be implemented in routine clinical practice. Diagnostic facilities like Next Generation Sequencing (NGS), despite being highly valuable for molecular diagnosis of specific PID disorders are not feasible in resource limited settings if applied imprudently to each and every patient with recurrent infections. The importance of clinical history and examination cannot be stressed enough and help guide the clinician from the clinical phenotype of a PID to a particular genetic defect [4].
Clinical approach to PID disorders involves answering 4 basic queries, namely ‘when to suspect a PID?’, ‘what category of PID from the IUIS classification does the clinical phenotype of the patient best fit?’, ‘what laboratory tests would help in evaluating and differentiating between the various PID disorders in the category selected?’ and ‘how to confirm a specific etiology?’.
PID should be suspected in a patient who fulfils one or more of the ten warning signs of PID disorders [51]. The next step would be to characterize the clinical phenotype of the patient giving special attention to the foci of infections, the microbes responsible and the response to treatment [11]. Based on the clinical phenotype of the patient, appropriate laboratory tests are performed as shown in Figure 2.
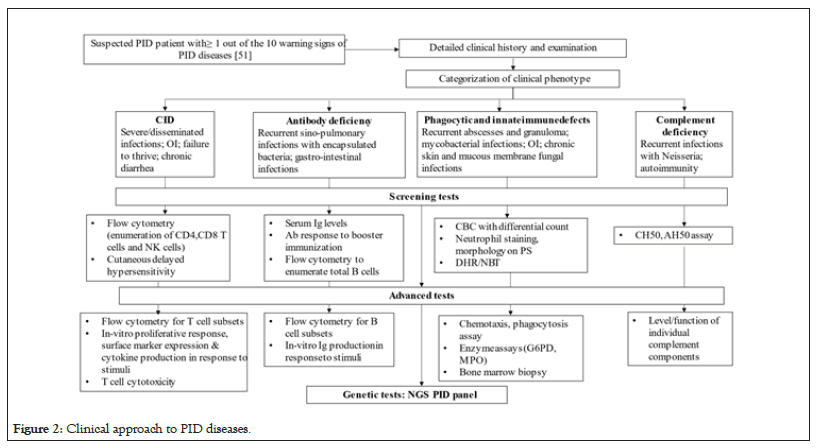
Figure 2: Clinical approach to PID diseases.
The Asia Pacific Society for Immunodeficiencies (APSID) has suggested diagnostic tests like estimation of serum immunoglobulin levels, flow cytometry for lymphocyte subset analysis, Nitro-Blue Tetrazolium (NBT) test, Dihydrorhodamine (DHR) test, Complement levels, Total and Alternative complement pathway hemolytic activity (CH50 and AH50) assay, functional antibody response to vaccines and Sanger sequencing to be made available in national or regional PID centres in medium Human Development Index (HDI) countries [8]. The ultimate aim would be to arrive at a molecular diagnosis for a multitude of reasons namely to unequivocally establish the diagnosis, to permit accurate genetic counselling, to plan future pregnancy and to identify candidates for gene-specific therapies [11].
Patients with HIGM syndromes present with a wide spectrum of clinical features even with identical genetic defects [52]. Most of the symptoms start in infancy/second year of life as recurrent bacterial and opportunistic infections. They are also prone for autoimmune disorders, haematological abnormalities, lymphoproliferation and malignancies.
HIGM Type 1 and 3 (CD40 L and CD40 deficiency)
The clinical and immunological features of CD40 deficiency are similar to CD40L deficiency albeit CD40 deficient B cells are unable to proliferate and undergo in-vitro CSR upon activation with CD40 agonists and cytokines [53]. The commonest form of HIGM syndrome comprising about 70% of patients with CSR defects is due to mutations in the gene encoding CD40L [28]. They become symptomatic in early life with a median age of diagnosis less than 12 months [16]. Upper and lower respiratory tract infections are the most common manifestations [33]and opportunistic infections caused by Pneumocystis jirovecii is frequently the initial presenting illness among infants. Cryptococcal infections are also common and CD40L deficiency is also associated with sclerosing cholangitis [54]. Bartonella, Herpes family viruses, Cryptosporidium parvum, Histoplasma, Mycobacterium avium, Leishmania and Candida albicans are some of the microbes causing opportunistic infections as per European and American cohorts [55,56].
Autoimmune complications frequently noted in HIGM 1 and 3 include seronegative arthritis and inflammatory bowel disease [33]. Coombs positive haemolytic anaemia, autoimmune hepatitis, hypothyroidism and discoid lupus erythematous are other autoimmune complications [57-59]. HIGM 1 patients are also more prone for malignancies affecting the biliary tree, gut and neuroendocrine tumours [33,34].
HIGM Type 2 (AID deficiency)
Recurrent sino-pulmonary infections are the hallmark of AID deficiency [60,61] with onset before two years of age. Other sites of infection include skin, lymph nodes, gastrointestinal tract and central nervous system [62]. Among CNS infections, meningitis due to Hemophilus influenza and Herpes simplex virus was noted [63]. Opportunistic infections are not a common feature of AID deficiency, clinically distinguishing HIGM 2 from HIGM 1 and 3.
Marked lymphoid hypertrophy is a typical manifestation of AID deficiency and is noted in half to one-third of cases [62,63]. Peripheral lymphadenopathy and tonsillar hypertrophy with giant germinal centres were most commonly reported [42,64], followed by hyperplasia of mesenteric lymph nodes, mediastinal lymph nodes, spleen, liver and tonsils [63]. Incidence of autoimmune complications is fairly high (20%), including autoimmune cytopenias, arthritis and hepatitis [62,63]. Even though there is lymphoid hyperplasia; malignant lymphoproliferations have not been described in HIGM 2.
HIGM Type 4
The clinical phenotype of HIGM 4 is similar to HIGM Type 2, but is generally milder. Sino-pulmonary infections, sepsis, lymphadenitis and osteomyelitis are commonly reported. HIGM 4 is rarely associated with myelodysplasia as well [65].
HIGM Type 5 (UNG deficiency)
Deficiency of UNG is another cause of HIGM syndrome in a very small number of patients [45] with clinical characteristics similar to HIGM 2, being susceptible to bacterial infections, lymphoid hyperplasia and autoimmunity [60].
HIGM Type 6 (NEMO and IKB1A mutation)
X linked anhidrotic Ectodermal Dysplasia with Immune-Deficiency (EDA-ID) is a HIGM caused by mutation of the NEMO/IKKy gene exclusively affecting males [66]. Affected boys have defective sweat glands, relatively thin hair, ectodermal dysplasia, dry scaly skin, dental abnormalities (conical shaped teeth) and suffer from a wide range of infections such as pneumonia, osteomyelitis, enteritis and meningitis [4].
Laboratory abnormalities and diagnosis of HIGM syndromes
All HIGM syndromes are associated with markedly reduced IgG, IgE and IgA levels, normal/elevated IgM levels, lack of antibody response to protein and polysaccharide antigens and are characterized by normal number of total B cells, but have grossly reduced number of memory (CD27+) B cells and absent switched memory (IgD-CD27+) B cells [67,68]. About two-thirds of HIGM 1 patients have neutropenia [33].
Diagnostic approach to HIGM syndromes begins with identification of the clinical phenotype as either combined or humoral immune-deficiency (Figure 2). Patients suspected of having HIGM based on clinical features and low IgG, IgE and IgA levels and normal/elevated IgM levels should undergo flow cytometry for lymphocyte subset analysis (T, B and NK cells) and for estimation of number of B cells in various stages of development (naïve, memory, switched memory B cells).
Confirmation of CD40L deficiency is achieved by demonstration of impaired CD40L expression on the surface of activated CD4+ T cells [69]. Agents like Phorbol Myristate Acetate (PMA) and ionomycin are used for in-vitro activation of T cells, following which they are stained with anti-CD40L monoclonal antibodies for identification of CD40L expression [69]. Staining for other T cell activation markers like CD69 and CD25 has to be done simultaneously to ensure proper in-vitro activation. Few mutations have been recognized in HIGM 1 patients which permit low level surface expression of non-functional CD40L on activated T cells in whom anti-CD40L monoclonal antibody staining would be normal [70]. In such patients whose clinical phenotype is strongly suspicious for CD40L deficiency, flow cytometry using biotinylated CD40-Ig chimeric construct or monoclonal antibodies against CD40-binding epitope should be carried out [70].
CD40 deficiency is confirmed by flow cytometry of peripheral blood mononuclear cells (B cells) which in the normal state constitutively express CD40 (doesn’t require in-vitro activation) and demonstrating the lack of surface expression of CD40 [71]. Final confirmation of both CD40L and CD40 deficiency requires genetic testing. AID and UNG deficiencies also require genetic testing for confirmation [72].
Management of hyper IgM syndromes
There are numerous therapeutic approaches to HIGM syndromes, including immunoglobulin replacement therapy, antimicrobial treatment, granulocyte-colony stimulating factor, CD40-agonist therapy, immunosuppressive therapy, stem cell therapy and gene therapy. Immunoglobulin replacement therapy is indicated for all HIGM patients in general, with additional therapies being available for each type of HIGM [73].
Treatment of CD40L/CD40 deficiency
Treatment of CD40L/CD40 deficiency, both being combined immune-deficiencies is complex and involves tackling multiple issues like prevention of opportunistic infections (notably Pneumocystis jiroveci and Cryptosporidium parvum) and management of neutropenia. For HIGM 1, the most effective therapeutic option is Hematopoietic Cell Transplantation (HCT). In a study done by Gennery et al., [74], among HIGM patients with and without organ damage like liver disease and bronchiectasis, 58% overall cure rate was achieved with HCT. Pre-transplant Reduced Intensity Conditioning (RIC) may improve outcomes with HCT but are associated with higher incidence of rejection and infection [75]. HCT at earlier age, absence of liver disease [76] and absence of lung disease [74] are some of the variables associated with better survival. Cryptosporidium parvum reactivation after HCT, either clinical or subclinical can be potentially fatal, thus warranting periodic surveillance by means of stool Polymerase Chain Reaction (PCR) [77]. HCT is less effective in autosomal recessive CD40 deficiency (HIGM Type 3) patients since it restores CD40 expression only for hematopoietic stem cell-derived cell lineages and not for other CD-40 expressing cell types [20]. Immunoglobulin replacement therapy is effective in preventing sino-pulmonary infections and bronchiectasis [76]. Prevention of opportunistic infections is a key aspect in the management of any combined immune-deficiency disease. Continuous cotrimoxazole prophylaxis is warranted for CD40L/CD40 deficiency patients [33]. Tackling Cryptosporidium parvum is a challenge and the best prophylactic strategy is not chemoprophylaxis with nitazoxanide or azithromycin (may reduce infection but long-term efficacy is unknown) but hygienic measures like drinking boiled water and avoiding swimming in lakes, ponds and non-chlorinated pools [33]. Neutropenia can be treated with Granulocyte- Colony Stimulating Factor (GCSF) [53] and liver transplant can be considered in patients with sclerosing cholangitis with liver failure [78]. Treatment with recombinant CD40L replacement and gene therapy for CD40L deficiency require further research before clinical application [79].
Treatment of AID deficiency
The mainstay of treatment of HIGM 2 is immunoglobulin replacement therapy. Early initiation of intravenous or subcutaneous immunoglobulin replacement at a dose of 400-600 mg/kg/month reduces the severity and frequency of sino-pulmonary infections, antibiotic use, hospitalisation rate, improves pulmonary function and quality of life [80,81]. HCT is not justified in HIGM 2 as they are pure humoral immune-deficiencies and have good response to immunoglobulin therapy [16]. Immuno-suppressive drugs like corticosteroids, cyclophosphamide and cyclosporine [63] can be considered in patients with autoimmune manifestations.
Treatment of HIGM 4,5 and 6 syndrome are same as that of HIGM 2 and include immunoglobulin replacement therapy and antibiotics [82,83].
Leniolisib, an oral small molecule that inhibits the active binding site of PI3K-delta has been recently approved by the US Food and Drug Administration for APDS patient’s ≥ 12 years of age, after the promising results of a small phase III trial [84].
Monitoring and prognosis of HIGM syndromes
Patients with CD40L/CD40 deficiency need regular monitoring of liver function every 4-6 months and ultrasound evaluation once a year. Stool PCR for Cryptosporidium and Microsporidium has to be done twice a year and prompt treatment with azithromycin or nitazoxanide should be started in case of positive test results.
Patients with AID and UNG deficiency should be monitored for the development of bronchiectasis and lymphoproliferative disease through periodic chest radiological imaging. Patients with HIGM 1 should avoid live virus vaccines like the varicella and oral polio vaccines due to the remote chance of developing infection with vaccine-strain viruses [85].
Prognosis
Prognosis of patients with CD40L/CD40 deficiency is guarded even with aggressive immunoglobulin replacement therapy and Pneumocystis prophylaxis [85]. Severe opportunistic infections, liver/biliary tract diseases and malignancies are common causes of mortality in these patients. Survival probability was approximately 60% at 20 years for those without liver disease versus 20% for those with liver disease [86]. The long-term prognosis of AID and UNG deficiency is comparatively better and regular use of immunoglobulin replacement therapy and prompt treatment of infections helps in avoiding development of chronic lung diseases and early death.
HIGM syndromes are highly under-diagnosed in low and middle-income countries owing to the lack of awareness among primary care physicians and limited diagnostic facilities. Knowledge of the various HIGM syndromes among clinicians would enable early initiation of appropriate treatment, thus significantly improving the quality of life of patients. Clinicians should strive to achieve the molecular diagnosis in each HIGM patient as long-term outcomes and treatment strategies may vary depending on the molecular defect.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Prakash PR, Gupta G, Adarsh AK, Sashank S, Sinha S (2023) Hyper IgM Syndromes: A Brief Review of the Pathogenesis, Clinical Features and Management. J Clin Cell Immunol. 14:693.
Received: 23-Jun-2023, Manuscript No. JCCI-23-25267; Editor assigned: 26-Jun-2023, Pre QC No. JCCI-23-25267 (PQ); Reviewed: 10-Jul-2023, QC No. JCCI-23-25267; Revised: 19-Jul-2023, Manuscript No. JCCI-23-25267 (R); Published: 27-Jul-2023 , DOI: 10.35248/2155-9899.23.14.693
Copyright: © 2023 Prakash PR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.