International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Original Research Article - (2022)Volume 10, Issue 2
Rationale: The hallmarks of pulmonary derangement due to severe COVID-19 disease include hypoxemia and a dysregulated and excessive immune response, i.e. a "cytokine storm". Several case series reported on the beneficial effect of Hyperbaric Oxygen Therapy (HBOT) on COVID-19 patients.
Objective: The aim of the study was to evaluate the effects of HBOT on COVID-19 patients.
Design: Randomized controlled design.
Setting: Single medical center with primary level care.
Participants: Thirty-one severe COVID-19 inpatients, suffering from respiratory insufficiency (saturation lower than 94% on room air or PaO2/FiO2<300 mmHg in addition to at least one risk factor) in addition to at least one other risk factor, were randomized between May-October 2020 to HBOT or a control arms in a 2:1 ratio. Patients underwent baseline evaluations which included symptoms questionnaire, vital signs, and blood tests.
Interventions: The HBOT arm patients underwent total of eight HBOT twice daily 1-hour sessions. The evaluation was repeated on day 5, the day after the last HBOT session.
Original primary endpoint was changed from arterial blood gas oxygenation to oxygen saturation, 5 days after enrollment. Secondary endpoints included vital signs, NEWS severity score, blood inflammatory markers, x-ray changes and outcomes.
Results: One day following the last HBOT session, there was a significant increase in room air saturation in the HBOT patients from 89.75 ± 2.67 to 93.78 ± 3.49, p<0.0014, compared to a non-significant decline in the control group from 90.44 ± 2.40 to 87.71 ± 7.86, p=0.356. HBOT group NEWS severity score improved from 5.94 ± 1.18 to 2.60 ± 2.10, p=0.001, while there was non-significant worsening in the control group 5.11 ± 1.36 to 5.71 ± 1.89, p=0.253. The respiratory rate decreased from 28.6 ± 5.5 to 20.1 ± 5.2 in the HBOT group (p<0.0001), compared to a non-significant increase in the control group from 25.1 ± 5.3 to 29.8 ± 6.7 (p=0.19). There was a significant decrease in CRP and LDH in the HBOT group compared to the control group and a significantly higher proportion developed COVID-19 IgG antibodies compared to the control group.
In the HBOT group, two patients experienced mild middle ear barotrauma and one patient suffered a myocardial infarction.
Conclusion: This study demonstrates, for the first time in a randomized clinical trial, that HBOT is a therapeutic modality that can improve oxygenation, attenuate inflammation, and improve the clinical status of severely-ill COVID-19 patients. Although underpowered, our study suggests the suggested HBOT protocol may be deployed safely with low rate of side effects. Larger scale studies are needed to evaluate the effect on inpatient mortality.
Hyperbaric oxygen; Therapy; COVID-19; Hypoxemia
COVID-19 isa ucsed by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which primarily targets the respiratory tract. In the disease state, hypoxemia is the hallmark of pulmonary derangement, and based on it and its related consequences, the disease is classified as mild (81%), severe (14%), or critical (5%) [1-3]. Interestingly, the direct cytopathic effect of the virus induces hypoxemic respiratory failure (type I respiratory failure) with impaired gas exchange and hypoxia without significant hypercapnia and with dyspnea that can be disproportionately mild relative to the severity of disease [4]. This severe and abrupt hypoxemia has often led to urgent intubation and mechanical ventilation, but without clinical-survival benefit, even with the additional use of extracorporeal membrane oxygenation [5-7]. Hypoxemia, may also play a crucial pathophysiological role by triggering a vicious cycle that includes a dysregulated and excessive immune response, also known as a "cytokine storm", which may further exacerbate systemic damage. Hypoxia may exacerbate the pro-inflammation effect [8]. The severity of the disease is reflected by high levels of C-reactive protein (CRP), ferritin, LDH and Interleukin-6 (IL-6) [9,10].
Currently, treatment options for COVID-19 infection include interventions that target viral replication (Remdesivir and antibodies), anti-inflammatory medications (steroids), in addition to supportive therapy such us anticoagulants and oxygen by nasal canula or mask [11-15]. Hyperbaric Oxygen Therapy (HBOT) includes breathing 100% oxygen in pressures higher than one Absolute Atmosphere (ATA), thus increasing the amount of oxygen dissolved in the plasma and in different tissues. HBOT is an FDA approved medical treatment with several indications including carbon monoxide poisoning, necrotizing fasciitis, compromised flaps, non-healing wounds, late effects of radiation therapy, and air diving decompression illness. In the past few months, several case series were reported on treating COVID-19 patients with HBOT. Following 1-8 HBOT sessions, in addition to the standard therapy, patients demonstrated symptomatic relief, increased oxygen saturation, and decreased inflammatory markers [16]. Gorenstein, et al. treated 20 COVID-19 patients and compared their results with a matched cohort. The treated patients had lower rates of mechanical ventilation [17].
The aim of the current study was to evaluate the effects of HBOT in severely ill hospitalized, non-intubated, COVID-19 patients during the acute early phase of the disease using a prospective randomized controlled design.
Study design
This was a randomized controlled trial among 30 severely ill COVID-19 patients admitted between May 1st, 2020, and October 15th, 2020, to the Shamir (Assaf-Harofeh) Medical Center, Israel. This study was approved by the Institutional Review Board (IRB) at Shamir Medical Center and the Israeli National Review Board. The study was registered at clinicaltrials.gov NCT04358926. The study was conducted and reported according to the CONSORTstatement.
Subjects
Non Intensive Care Unit (ICU) hospitalized patients 18 years or older with a SARS-CoV-2 RT-PCR-confirmed diagnosis of severe COVID-19 infection were eligible for enrollment. Patients had to have respiratory insufficiency of either room saturation lower than 94% on room air or PaO2/FiO2<300 mmHg in addition to at least one risk factor (hypertension, moderate-severe asthma, diabetes mellitus, cardiac conditions, severe obesity (BMI>40), age>65, immunodeficiency, chronic liver disease). Included patients were on respiratory support of any kind. Patients were excluded if they were pregnant, had a pneumothorax, pneumomediastinum, claustrophobia, ear/sinus diseases which aren't allowed in HBOT, severe emphysema or known pulmonary bullae, or an inability to sign the informed consent.
Suitable candidates among inpatient admissions were offered to participate in the study. A physician certified in hyperbaric therapy provided a consultation to evaluate patients for eligibility.
Following signing an informed consent, patients were randomized using simple randomization to either HBOT or control arms in a 2:1 ratio according to a randomization table, supervised by a researcher which informed the in-unit investigators of the patient group allocation. Following randomization, all patients underwent baseline evaluations which included a symptoms questionnaire, vital signs, and blood tests. The HBOT arm patients underwent eight HBOT sessions twice daily. The evaluation was repeated on day 5, the day after the last HBOT session.
Interventions
The HBOT protocol was administrated in a Monoplace chamber BLKS-303 model (Khrunichev State Research and Production Space Center, Russia), located withing the COVID-19 unit. The protocol comprised of eight consecutive sessions, two sessions per day, four days consecutively. Each session included breathing 100% oxygen at 2.2 Absolute Atmospheres (ATA) for 60 minutes with no air breaks. Compression/decompression rates were 0.1 ATA/ minute. All sessions were supervised by a hyperbaric medicine trained physician and hyperbaric trained nurse at all times.
The control group received standard medical treatment. The prescribed medical treatment for both arms were continued during the study. This included Remdesivir, COVID-convalescent plasma therapy oxygen, steroids, antibiotics, and Low-Molecular Weight Heparin (LMWH).
Endpoints
Primary endpoint was oxygen saturation 5 days after enrollment (see below). Secondary endpoints included heart rate, blood pressure, respiratory rate, NEWS severity score, inflammatory markers (CRP, LDH, Ferritin) and 30-days outcomes. SARS-CoV-2 Serology was added during post-hoc analysis.
Vital signs monitoring
Heart rate, blood pressure, oxygen saturation (via pulse oximeter) and respiratory rate were measured at baseline, prior to each HBOT session, following each HBOT session and one day following the last HBOT session. The National Early Warning Score (NEWS) score was calculated [18]. When a patient’s medical condition did not allow room air saturation measurements, the patient’s score was not included in the analysis.
Arterial blood gasses
The ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen at 5 days after enrollment was determined as the primary endpoint of the study. However, the ability to draw arterial blood gases with full COVID-19 protection gear was found to be challenging more than usual inconvenient to the patients and many of the patients asked to avoid it (especially the draw of second arterial blood gas). Therefore, this endpoint was not completed and changed from the original protocol to oxygen saturation.
Blood samples
Whole blood samples were collected into EDTA and gel tubes using a standard technique at inclusion, and one day following the last HBOT session. Complete blood count and chemistry tests were performed by standard protocols. Upon collection, samples were stored at −80°C. At the completion of the study, blood samples were defrosted, and serology was assessed. All laboratory personnel were blinded to participant characteristics and clinical information.
SARS-CoV-2 serology
COVID-19 serology tests were performed using the LIAISIN SARSCoV- 2 IgM (REF311470, DiaSorin) and LIAISIN SARS-CoV-2 S1/S2 IgG (REF311450, DiaSorin). Serology kits were used in accordance with the manufacturer instructions. In short, A specific antigen is used for coating magnetic particles (solid phase). During the first incubation, the SARS-CoV2 IgM/IgG antibodies present in calibrators, samples, or controls, bind to the solid phase. During the second incubation, mouse monoclonal antibodies to human IgM linked to an isoluminol derivative (isoluminol-antibody conjugate), react with SARS-CoV2 IgM already bound to the solid phase. After each incubation, the unbound material is removed with a wash cycle. Subsequently, the starter reagents are added and a flash chemiluminescence reaction is thus induced. The light signal, and hence the amount of isoluminol-antibody conjugate, is measured by a photomultiplier as Relative Light Units (RLU) and indicates the presence or absence of antibodies to SARS-CoV2 present in calibrators, samples, or controls.
Chest X-rays
Chest X-rays were taken using a mobile unit in the COVID-19 department at baseline and 1-2 days after the last intervention. Films were assessed by a blinded radiologist who compared the films for findings improvement, deterioration, or no changes.
30-day outcomes
Thirty-day outcomes were obtained from medical records including time to negative COVID-19 PCR, hospitalization length, mortality, and the need for mechanical ventilation.
Other predefined endpoints
Spirometry proved to be unobtainable in the COVID-19 department setting. IL-1,IL-2,IL-6,IL-10, TNF-alpha, procalcitonin results were not included.
Patient and public involvement
Patients were not involved in the design and conduct of this research. We carefully assessed the burden of the trial interventions on patients. We intend to disseminate the main results to trial participants and will seek patient and public involvement in the development of an appropriate method of dissemination.
Statistical analysis
The normal distribution for all variables was tested using the Kolmogorov-Smirnov test. Unless otherwise stated, continuous data are expressed as means ± standard deviation and compared by independent t-tests. Categorical data are expressed in numbers and percentages and compared by chi-square/Fisher’s exact tests. To evaluate HBOT’s effects on room air saturation, a within-subject repeated measures ANOVA model was used to test the main interaction effect between time and group. Significance level was determined as 0.05. False discovery rate was performed to control for multiple comparisons. Analysis was performed using MATLAB 2019b (MathWorks).
Sample size
Based on a previous study on HBOT effect on ARDS [19], with 95% power and significance level of 5%, and a groups difference of 30 (SD 15-18) in the PaO2/FaO2 and a 2:1 group allocation, the sample requires 7 NBOT patients and 13 HBOT patients. Considering 15% dropout, we will recruit 10 NBOT patients and 20 HBOT patients, 30 in total.
Thirty-one individuals were assessed for HBOT eligibility. Out of which, 29 patients were randomized and two patients had no respiratory insufficiency upon hyperbaric physician assessment. Three patients discontinued the HBOT sessions and one additional patient did not complete his post-HBOT assessment. Due to the complexity of access to these patients, dropped out patients outcomes were not obtained. Twenty-five patients were included in the final analysis (Figure 1). The mean age was 65.44 ± 7.8, and 60% were males. There were no significant differences in demographics, time lapse between hospitalization to randomization, high risk conditions or medical treatment between the two arms (Table 1). At baseline, there were no significant differences between the two groups in vital signs including room air saturation, heart rate, diastolic blood pressure and respiratory rate (Table 2). No patient was intubated at baseline.
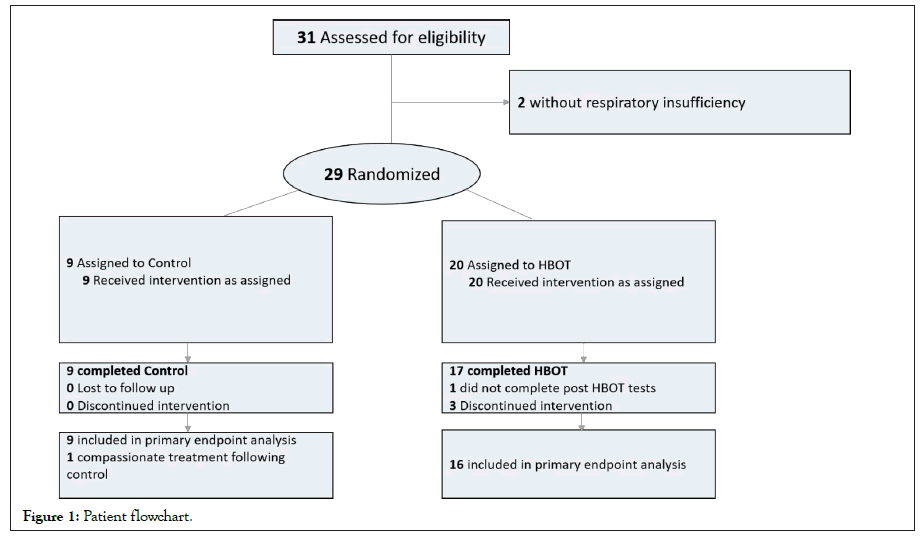
Figure 1: Patient flowchart.
| Total | HBOT | Control | Sig. | |
|---|---|---|---|---|
N |
25 | 16 | 9 | |
Age (years) |
65.44 ± 7.8 | 64.25 ± 7.8 | 67.56 ± 7.8 | 0.32 |
Males |
15 (60%) | 10 (62.5%) | 5 (55.6%) | 0.73 |
BMI |
29.36 ± 3.3 | 29.18 ± 3.3 | 29.69 ± 3.4 | 0.72 |
Time lapse from hospitalization to randomization |
2.04 ± 2.05 | 2.18 ± 2.4 | 1.77 ± 1.30 | 0.642 |
High risk conditions |
||||
Obesity |
2 (8%) | 1 (6.3%) | 1 (11.1%) | 1F |
Cancer |
1 (4%) | 0 | 1 (11.1%) | 0.36F |
Diabetes mellitus |
15 (60%) | 8 (50.0%) | 7 (77.8%) | 0.17 |
Hypertension |
18 (72%) | 12 (75%) | 6 (66.7%) | 0.65 |
Heart disease |
6 (24%) | 3 (18.8%) | 3 (33.3%) | 0.63F |
Immune deficiency |
0 | 0 | 0 | 0 |
Asthma |
2 (8%) | 1 (6.3%) | 1 (11.1%) | 1F |
Chronic lung disease (non-asthma) |
0 | 0 | 0 | 0 |
Chronic liver disease |
0 | 0 | 0 | 0 |
Chronic kidney disease |
0 | 0 | 0 | 0 |
Treatment |
||||
Steroids |
23 (92%) | 16 (100%) | 7 (77.7%) | 0.14 |
Antibiotics |
14 (56%) | 10 (62.5%) | 4 (44.4%) | 0.33 |
Antiviral |
17 (68%) | 11 (68.8%) | 6 (66.7%) | 0.5 |
Plasma |
20 (80%) | 13 (81.3%) | 7 (77.88%) | 0.37 |
Anti IL-6 |
6 (24%) | 5 (31.3%) | 1 (11.1%) | 0.24 F |
Enoxaparin |
18 (72.%) | 13 (81.3%) | 5 (55.6%) | 0.17 |
Table 1: Baseline characteristics.
At the end of the study protocol, one day following the last HBOT session, there was a significant increase in room air saturation in the HBOT group (N=14) from 89.75 ± 2.67 to 93.78 ± 3.49, p<0.0014, compared to a non-significant decline in the control group (N=7) from 90.44 ± 2.40 to 87.71 ± 7.86, p=0.356. There was a significant group by time interaction post-HBOT compared to the control group (F=7.109, p=0.015) (Table 2 and Figure 2). The respiratory rate decreased significantly from 28.6 ± 5.5 to 20.1 ± 5.2 in the HBOT group (p<0.0001), compared to a non-significant increase in the control group from 25.1 ± 5.3 to 29.8 ± 6.7 (p=0.19). There was a significant group by time interaction post-HBOT compared to the control group (F=15.269, p=0.001).
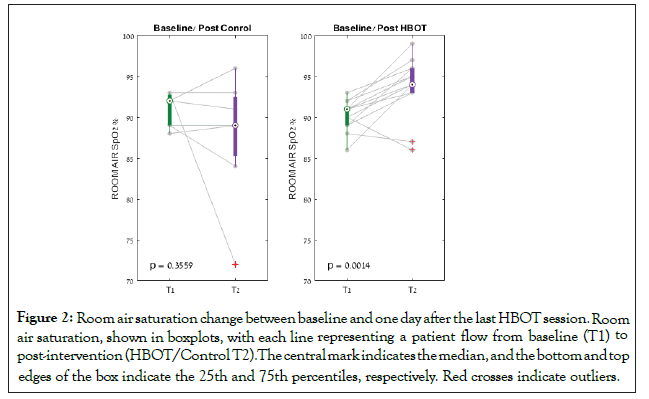
Figure 2: Room air saturation change between baseline and one day after the last HBOT session.Room air saturation, shown in boxplots, with each line representing a patient flow from baseline (T1) to post-intervention (HBOT/Control T2).The central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. Red crosses indicate outliers.
| Measurements | HBOT Baseline | Control Baseline | Sig.* | FDR | Post-HBOT | Sig. ** | FDR | Post-Control | Sig.** | FDR | Interaction Sig. *** | FDR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vital Signs | ||||||||||||
| N | 16 | 9 | 14 | 7 | ||||||||
| Room air saturation | 89.75 ± 2.67 | 90.44 ± 2.40 | 0.356 | 0.427 | 93.78 ± 3.49 | 0.0014 | 0.0056 | 87.71 ± 7.86 | 0.355 | 0.426 | 0.015 | 0.03 |
| N | 15 | 7 | 15 | 7 | ||||||||
| NEWS | 5.86 ± 1.18 | 5.0 ± 1.52 | 0.16 | 0.316 | 2.6 ± 2.09 | <0.0001 | <0.0001 | 5.71 ± 1.89 | 0.253 | 0.426 | 0.001 | 0.003 |
| Systolic BP | 133.9 ± 16.5 | 120.0 ± 12.4 | 0.035 | 0.183 | 129.2 ± 18.9 | 0.248 | 0.426 | 128.1 ± 21.1 | 0.343 | 0.426 | 0.122 | 0.183 |
| Diastolic BP | 76.2 ± 16.8 | 64.0 ± 10.8 | 0.061 | 0.183 | 74.9 ± 13.8 | 0.777 | 0.847 | 64.4 ± 5.7 | 0.9 | 0.9 | 0.788 | 0 |
| Heart rate | 79.7 ± 16.0 | 81.8 ± 11.2 | 0.777 | 0.777 | 75.9 ± 11.8 | 0.323 | 0.426 | 72.2 ± 22.3 | 0.095 | 0.285 | 0.345 | 0.414 |
| Respiratory rate | 28.6 ± 5.5 | 25.1 ± 5.3 | 0.211 | 0.3165 | 20.1 ± 5.2 | <0.0001 | <0.0001 | 29.8 ± 6.7 | 0.19 | 0.426 | 0.001 | 0.003 |
| Complete Blood Count | ||||||||||||
| WBC | 6.64 ± 2.35 | 5.42 ± 3.10 | 0.277 | 0.289 | 9.13 ± 3.22 | 0.02 | 0.06 | 8.82 ± 5.77 | 0.111 | 0.204 | 0.635 | 0.635 |
| HB | 13.69 ± 1.46 | 13.00 ± 1.66 | 0.289 | 0.289 | 12.86 ± 3.74 | 0.327 | 0.392 | 12.77 ± 1.56 | 0.465 | 0.465 | 0.602 | 0.635 |
| Platelets | 242.06 ± 85.89 | 205.11 ± 54.42 | 0.258 | 0.289 | 285.00 ± 106.65 | 0.136 | 0.204 | 301.67 ± 90.60 | 0.004 | 0.02 | 0.202 | 0.605 |
| Inflammatory markers£ | ||||||||||||
| CRP-B | 106.52 ± 18.46 | 122.67 ± 27.55 | 0.618 | 0.618 | 26.62 ± 5.95 | 0.001 | 0.006 | 106.14 ± 21.53 | 0.427 | 0.512 | 0.032 | 0.048 |
| LDH | 695.4 ± 25.67 | 510.00 ± 16.93 | 0.021 | 0.063 | 522.13 ± 26.19 | 0.004 | 0.012 | 550.00 ± 18.93 | 0.63 | 0.63 | 0.027 | 0.048 |
| Ferritin | 1597.00 ± 228.32 | 1493.56 ± 333.85 | 0.08 | 0.12 | 771.87 ± 54.94 | 0.045 | 0.09 | 975.67 ± 160.5 | 0.28 | 0.42 | 0.611 | 0.611 |
| Chemistry£ | ||||||||||||
| Globulin | 31.73 ± 0.84 | 30.89 ± 0.58 | 0.052 | 0.838 | 28.00 ± 0.46 | 0.005 | 0.065 | 28.33 ± 0.51 | 0.029 | 0.137 | 0.475 | 0.686 |
| Albumin | 34.67 ± 0.47 | 34.67 ± 0.33 | 0.853 | 0.963 | 34.33 ± 0.80 | 0.799 | 0.865 | 31.11 ± 0.61 | 0.034 | 0.137 | 0.119 | 0.386 |
| Protein | 66.47 ± 1.03 | 65.56 ± 0.89 | 0.017 | 0.221 | 60.53 ± 0.64 | 0.001 | 0.026 | 59.22 ± 0.96 | 0.015 | 0.09 | 0.87 | 0.87 |
| Uric Acid | 4.51 ± 0.16 | 4.83 ± 0.18 | 0.919 | 0.963 | 4.47 ± 0.12 | 0.856 | 0.89 | 4.02 ± 0.17 | 0.055 | 0.178 | 0.06 | 0.386 |
| Urea | 37.65 ± 2.27 | 46.00 ± 3.07 | 0.278 | 0.899 | 43.03 ± 1.11 | 0.177 | 0.511 | 52.64 ± 4.91 | 0.4 | 0.65 | 0.868 | 0.87 |
| ALT | 43.60 ± 5.00 | 23.22 ± 2.16 | 0.963 | 0.963 | 44.29 ± 5.43 | 0.746 | 0.865 | 23.00 ± 1.78 | 0.97 | 0.97 | 0.802 | 0.87 |
| AST | 53.29 ± 5.32 | 37.22 ± 2.56 | 0.639 | 0.963 | 46.86 ± 4.20 | 0.676 | 0.836 | 28.11 ± 1.02 | 0.3 | 0.557 | 0.726 | 0.87 |
| Na | 138.67 ± 0.67 | 137.22 ± 0.57 | 0.298 | 0.899 | 137.00 ± 0.46 | 0.267 | 0.552 | 139.89 ± 0.64 | 0.26 | 0.0.552 | 0.099 | 0.386 |
| K | 4.35 ± 0.05 | 4.51 ± 0.06 | 0.605 | 0.963 | 4.27 ± 0.05 | 0.492 | 0.752 | 4.14 ± 0.06 | 0.015 | 0.097 | 0.086 | 0.386 |
| Alk. Phos | 68.60 ± 2.71 | 77.44 ± 3.34 | 0.346 | 0.899 | 62.27 ± 2.09 | 0.037 | 0.137 | 83.33 ± 6.65 | 0.645 | 0.836 | 0.237 | 0.616 |
| Glucose | 156.87 ± 7.99 | 159.22 ± 5.41 | 0.769 | 0.963 | 149.87 ± 9.28 | 0.578 | 0.834 | 172.00 ± 10.60 | 0.633 | 0.836 | 0.444 | 0.686 |
| Creatinine | 0.79 ± 0.02 | 1.52 ± 0.26 | 0.568 | 0.963 | 0.75 ± 0.02 | 0.274 | 0.552 | 0.98 ± 0.08 | 0.399 | 0.65 | 0.287 | 0.621 |
| Calcium | 8.58 ± 0.06 | 8.64 ± 0.04 | 0.82 | 0.963 | 8.55 ± 0.04 | 0.789 | 0.865 | 8.43 ± 0.08 | 0.276 | 0.552 | 0.407 | 0.686 |
Note: *Two-sample t-test; **Paired t-test; *** Group X Time ANOVA; BP: Blood Pressure; £-mean ± SEM
Table 2: Changes in vitals and blood tests.
With respect to the NEWS severity score, at baseline, both groups were similar Table 2. However, at the end of the study, one day following the last HBOT, there was a significant improvement in the HBOT group’s NEWS score (N=15) from 5.94 ± 1.18 to 2.60 ± 2.10, p=0.001, while there was non-significant worsening in the control group (N=7) from 5.11 ± 1.36 to 5.71 ± 1.89, p=0.253. There was a significant group by time interaction post-HBOT compared to the control group (F=16.379, p=0.001) (Figure 3).
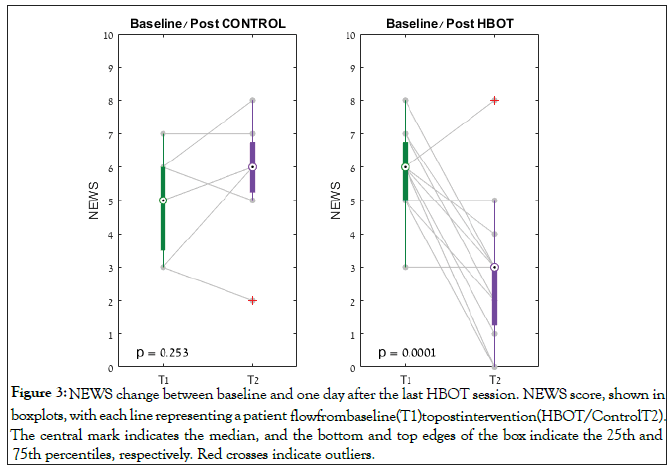
Figure 3: NEWS change between baseline and one day after the last HBOT session. NEWS score, shown in boxplots, with each line representing a patient flow from baseline (T1) to post intervention (HBOT/Control T2). The central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. Red crosses indicate outliers.
At baseline, there were no significant differences in the inflammatory markers, CRP-B, LDH and Ferritin (Table 2). One day following HBOT, there was a significant decrease in CRP in the HBOT group compared to the control group (F=5.322, p=0.032), (Table 2 and Figure 4). There was a significant decrease in Ferritin in the HBOT group, however the Group X time interaction was not significant (F=0.266, p=0.611) (Table 2). There was a significant decrease in LDH in the HBOT group compared to a non-significant increase in the control group (F=5.599, p=0.027). There were no other significant changes in the chemistry results (Table 2).
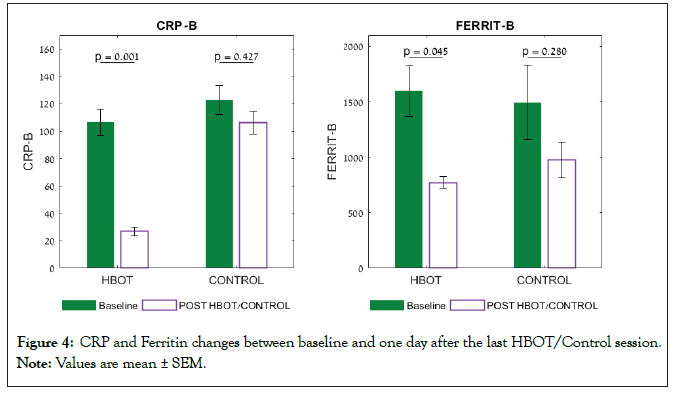
Figure 4: CRP and Ferritin changes between baseline and one day after the last HBOT/Control session.
Note: Values are mean ± SEM.
Using the DiaSorin-LIAISIN serology kit, there were no significant differences in SARS-CoV-2 IgG positive serology between the groups at baseline (56% vs. 60%, p=0.567) (Table 3). One day following the last HBOT session, there was a significant increase in IgG positive serology in the HBOT group compared to the control group (93% vs. 78%, p=0.003). In the IgM serology there was a significant difference between the groups’ positive percentages at baseline (80% vs. 67%, p<0.001) and one day post HBOT (100% vs. 80%, p<0.001).
| SARS-COV-2 Antibody | Baseline control (Positive%) | Baseline HBOT (Positive%) | Sig. | FDR | Post control (Positive%) | Post HBOT (Positive%) | Sig. | FDR |
|---|---|---|---|---|---|---|---|---|
| IgG-DiaSorin LIAISIN | 56 | 60 | 0.567 | 0.567 | 78 | 93 | 0.003 | <0.001 |
| IgM-DiaSorin LIAISIN | 67 | 80 | <0.001 | <0.001 | 89 | 100 | <0.001 | <0.001 |
Table 3: COVID-19 serology results.
Following HBOT, five patients (31.1%) had improved radiologic findings compared to none in the control group (0%) (p=0.07) (Figure 5). Worse radiologic findings were seen in 25% of HBOT patients compared to 60% in the control group (p=0.07) (Figure 5).
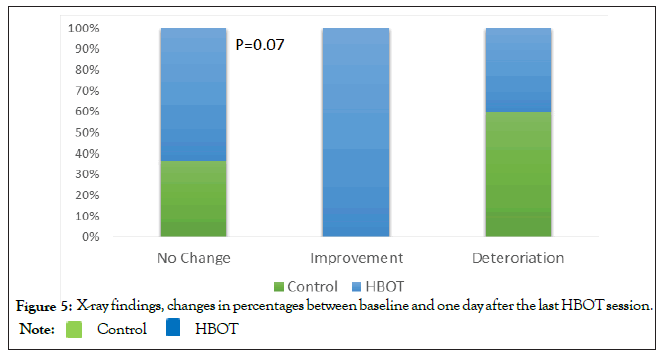
Figure 5: X-ray findings, changes in percentages between baseline and one day after the last HBOT session.

One patient from the control group received compassionate HBOT, as a rescue prior to mechanical ventilation and was removed from the 30 days analysis. No significant differences between the two groups were noted in hospitalization length, mortality and mechanical ventilation at 30 days (Table 4). There was a significant decrease in the time of a negative COVID-19 PCR in the HBOT group compared to the control group (19.91 ± 8.7 vs. 29.43 ± 8.7, p=0.034).
| Total | HBOT | Control | Sig | FDR | |
|---|---|---|---|---|---|
| N | 25 | 16 | 9 | ||
| Discharge | 20 (60.0%) | 13 (81.3%) | 7 (77.8%) | 0.83 | 0.83 |
| Mechanical ventilation | 6 (24.0%) | 3 (18.8%) | 3 (33.3%) | 0.41 | 0.65 |
| Mortality | 4 (16.0%) | 2 (12.5%) | 2(22.2%) | 0.52 | 0.65 |
| Hospitalizations length | 13.68 ± 6.8 | 12.46 ± 5.1 (N=13) | 16.33 ± 9.5 (N=6) | 0.65 | |
| Time to Negative Covid-19 PCR | 23.42 ± 9.7 | 19.91 ± 8.7 (N=12) | 29.43 ± 8.7 (N=7) | 0.034 | 0.17 |
Table 4: 30-days outcomes.
Safety
Two patients (12.5%) experienced mild middle ear barotrauma (TEED 1-2) in the HBOT group compared to none in the control group. One patient from the HBOT arm had a myocardial infarction and had percutaneous coronary intervention one day post HBOT. No other adverse events were reported.
The current randomized controlled study in severely ill COVID-19 patients demonstrated that a relatively short HBOT protocol can significantly improve room air saturation and NEWS severity score and attenuate inflammation as evaluated by CRP and LDH. In addition, there was a significant improvement in the chest radiology findings in the HBOT group as compared to the control. To the best of our knowledge, this is the first randomized controlled trial assessing the effect of HBOT in COVID-19 patients.
Previous literature
The use of HBOT for viral infection pandemics has been documented in the 1920s, when Dr. Cunningham used an hyperoxic chamber to treat severe Spanish influenza cases. He observed significant improvement in patients who were cyanotic and comatose [20]. A hundred years later, the world is once again is facing a new viral pandemic without an effective treatment so far. Two previous case series have suggested that HBOT is an effective treatment for severe COVID-19 patients. Chen, et al. reported a case series of five severe COVID-19 patients treated with 3-8 HBOT sessions in addition to the standard therapy. In all cases, they reported an increase in oxygen saturation, arterial oxygen content, lactate levels reduction, fibrinogen levels decrease and increases in lymphocyte numbers. Thibodeaux et al. used 1-6 sessions of 90 minutes of 100% oxygen at 2 ATA without air breaks, in five COVID-19 patients with similar results of symptom relief, decreases in inflammation markers and oxygen saturation improvement. Gorenstein, et al. treated 20 patients with five sessions of oxygen at 2 ATA for 90 minutes without air breaks, which were compared to a retrospective matched cohort, and found decreased mechanical ventilation rates. Our study used a randomized controlled design, and to reduce oxygen toxicity effects, the protocol used was composed of oxygen at 2.2 ATA for 60 minutes. The current study did not have the adequate sample size power to address either mortality or need for mechanical ventilation as primary or secondary end points.
Mechanisms of HBOT
The current findings may be explained by the known physiological effects of HBOT related to the SARS-CoV-2 virus pathogenesis. First, it has been recently postulated the SARS-CoV-2 binds to the heme component in the hemoglobin molecule and reduces oxygen’s affinity to hemoglobin [21]. During HBOT, the increased amount of available oxygen molecules increases the binding to the hemoglobin molecules. HBOT has shown significant beneficial effects in other competitive molecules such as carbon monoxide intoxication [22,23].
Second, during HBOT, the oxygen content in the different tissues increases 25-30 fold. This effect has two potential therapeutic aspects. By increasing the FiO2 significantly, we hypothesize the pulmonary oxygen gradient can overcome the inflammation in the alveoli and the thickened fibrosis caused by ARDS. Additionally, during HBOT, the amount of oxygen dissolved in the plasma becomes significant and enables sufficient tissue oxygenation without the need of red blood cells [24].
Third, HBOT is known to reduce both the protein levels and gene expression (mRNA) of the following inflammatory cytokines: IL-2, TNF-α, IL-6, IL-1β [25-27]. The anti-inflammatory effect of HBOT has been shown in chronic diseases as well as in models of acute infection and massive hemorrhage [28]. In addition, HBOT has immune modulation effects including improving the antioxidant activity of leukocytes, leukocyte adhesion and function, calcium homeostasis and platelets activation and aggregation [28].
In conclusion, this study demonstrates for the first time in a prospective randomized clinical trial, that HBOT can improve oxygenation, attenuate inflammation, and improve the clinical status of severely ill COVID-19 patients. Although underpowered, our study suggests,that the suggested HBOT protocol may be deployed safely with low rate of side effects. Thus, where available, HBOT can be added to the arsenal of therapeutic interventions in acute COVID-19 patients suffering from significant hypoxia. Larger studies are required to evaluate the effect on outcomes.
Larger scale studies are needed to better evaluate HBOT’s effect on inpatient mortality.
The limitations of the study are its small sample size and lack of a SHAM/placebo group. The original RCT protocol was supposed to be a double-blind design of a SHAM treatment composed of breathing 100% oxygen at 1 ATA in the monoplace. However, the mobilization of those severely ill patients from their hospitalization bed to the hyperbaric chamber and its compression was found to be very challenging involving desaturations and shortness of breath. Accordingly, after a pilot evaluation on one patient who suffered a dangerous desaturation, the control protocol was revised. Therefore, blinding and a true SHAM arm seem impractical to perform in those severely ill COVID-19 patients. Second, the study did not include long term measurements of the parameters and the sample size was underpowered to evaluate mortality. Third, the current study used eight HBOT sessions in four days even though the optimal HBOT protocol should be individualized and based on the patients' clinical condition and should be continued as long as he/she suffers from severe hypoxia.
• The use of HBOT in COVID-19 patients was evaluated in a randomized controlled design.
• The main limitation of the study lies in its small sample size.
• An additional limitation is the inability to obtain a SHAM/ placebo group.
• The prescribed HBOT protocol is not optimal and may have benefited from individualization per patient’s hypoxia.
The study was registered at clinicaltrials.gov NCT04358926.
We would like to thank the medical staff of Shamir medical center in their support of the study and their continuous outstanding efforts in these times.
HA, LZKO and ES conceptualized and designed the study, FS, AHR, CK, GL, KAV, TN, MH, WZ, LE, ZY, BY, NRL,MY, SY, GO, TI, ID, MA, SE treated the patients and acquired the data. AHR acquired the laboratory data. HA, MC and ES performed the results analysis. HA, MC, ES, SY, GO, IE, MA, LZKO and SE interpreted the data. HA, MC and ES prepared the manuscript. All authors reviewed the manuscript.
AH, ES, ZW, BY, ZY work for AVIV Scientific LTD. ES is a shareholder at AVIV Scientific LTD.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’.
Data are available upon reasonable request.
This research protocol was approved by the Shamir Medical Center IRB, ID 120-20-ASF.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Hadanny A, Finchi S, Catalogna M, Abu Hamed R, Korin C, Levi G et al. (2022) Hyperbaric Oxygen Therapy for COVID-19 Patients: A Prospective, Randomized Controlled Trial. Int J Phys Med Rehabil. 10:624.
Received: 10-Feb-2022, Manuscript No. JPMR-22-15839; Editor assigned: 14-Feb-2022, Pre QC No. JPMR-22- 15839 (PQ); Reviewed: 28-Feb-2022, QC No. JPMR-22-15839; Revised: 03-Mar-2022, Manuscript No. JPMR-22- 15839(R); Published: 10-Mar-2022 , DOI: 10.35248/2329-9096-22.10.624
Copyright: © 2022 Hadanny A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.