Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 6
Purslane is a vegetarian source of n-3 PUFA (α-linolenic acid). The objective of the present work was to explore purslane as n-3 source to mitigate hyperlipidemia in rat model. Group of rats (n=48) were fed on high cholesterol diet to induce hyperlipidemia. Group 1,2,3,4 and 5 (n=6/group) were fed on aqueous extract of purslane (AEP) and lovastanin diet while group 6 and 7 were treated as hyperlipidemic and normal control. The results revealed a significant (p<0.05) reduction in serum TC, LDL-C, VLDL-C, TAG, increase in serum HDL-C level observed in AEP fed rats as compared to cholesterol induced hyperlipidemic control group. Groups treated with AEP and lovastatin also demonstrated considerable decrease in LDL-C: HDL-C ratios, levels of SGOT, SGPT, ALP activities and body weight in comparison to control group. Therefore, administration of AEP concluded that 150 mg/kg body weight AEP is a potent cardio-protective agent having preventive and curative effect against hyperlipidemia.
Keywords: Purslane; Hypolipidemia; Drying; PUFA; Cardioprotective agent
The current lifestyle with high fat diet and less physical activity notably contribute to hypercholesterolemia and cardiovascular diseases [1]. Oxidative stress caused by reactive oxygen species (ROS) also plays main role in the etiology of numerous diseases including atherosclerosis and coronary heart disease [2]. Hyperlipidemia is one of the risk factors for coronary heart diseases. Epidemiological evidence supports that polyphenolic compounds found in fruit, vegetables and other plant material reduce coronary heart disease complications. Studies indicate that plant phenolics, flavonoids, flavonolignans and phenolic acids act at a molecular level as antioxidants and exhibits health-promoting properties. Furthermore, there are data suggesting the capability of phenolics to modulate and positively affect lipoprotein metabolism. Clinical trials also suggest that plant-based phenolics play a beneficial role in the prevention of coronary heart disease. Hence for herbal based cures is general practice. Hence nutraceuticals which can able to reduce hyperlipidemia induced complication or to control serum cholesteroland triacylglycerol levels has gained significant importance over the years, studied hypolipidemic and hepato-protective effect of n-3 and n-6 fatty acids rich flax and pumpkin seed lipids in hypercholesterolemic rats [3]. Although plant extracts compose potential candidates like phenolics they often have complexed with other molecules including antioxidants and pro-oxidant properties [4]. Owing to this reason in the present extract of purslane was investigated for its hypolipidemic potential. Reduction of the level of cholesterol in plasma is an efficient way to treat atherosclerosis. There are chemical drugs available (fibrates and statins) which has lipid-lowering potential. However, they fail to meet the demands for treatment further, patients prepare natural treatment rather synthetic drug due to their adverse effects and great drug dependence [5]. Hence, plant materials and their extracts became popular in treating hyperlipidemia with no adverse effects. Purslane, a member of Portulacaceae family, is well-known weed and has been ranked the eight mainly common plants in the world [6]. It has been reported to be the richest plant source of omega-3 fatty acids [FA] yet examined. Subsequent reports have confirmed the high levels of n-3 fatty acids and traces of EPA (C22: 5) and DHA (22: 6) [7] whose concentration is the highest in the leaves among leafy vegetables. Glutathione, glutamic acid and aspartic acid were also found in purslane. It is reported as the super food of the future due to its high nutritive and anti-oxidant properties [8]. It is considered as vegetable for long life in China and sold in theshops of United Arab Emirates and Oman. It is used as a traditional Chinese herbal medicine for skin [9]. A wide range of other pharmacological effects of purslane, such as antibacterial, analgesic, anti-inflammatory, wound-healing activities present in purslane. Anti-diabetic effect of extracts from purslane as alternate in streptozotocin induced diabetic rat model has also been reported [10]. Hypercholesterolemia has been reported to induce oxidative stress in various organs like liver, heart and kidney [11]. It has been shown that plants bio-actives antioxidants are better and random potential role in protecting people from numerous illnesses such as cardiovascular diseases. Purslane has also been reported to have a multiplicity of biological effects including hypoxia, hepatoprotective properties and anti-hypertension effect, however, scientific validation on its efficacy is scarce in the literature. Clinical studies proposed that dyslipidaemiais one of the most important risk factors for coronary disease. It was reported from the preclinical studies that hypercholesterolemia promotes accumulation of low-density lipoprotein in the arterial wall, endothelial cell dysfunction and expansion of atherosclerosis [12]. It is evident from the literature there is no systematic similar studies available, hence based on the available information, it is hypothesized that extract of purslane may ameliorate hypercholesterolemic condition by affecting on various biochemical markers responsible for hypercholesterolemia in a rat model. Therefore, this study attempts to appraise the hypocholesteromic effect of purslane extract. The outcome of the study will have implications for improving hypercholesteromic condition and purslane serve as partial replacement of anti-hyperlipidemic drug which causes side effects.
Plant material and preparation of purslane leaf extracts
Fresh purslane was procured from super market Mysore India. The leaves were collected and surface sterilized after washing thoroughly in tap water to remove adhering dust and mud particles, followed by rinsed in distilled water and the excess water was drained off. The dried leaves were stored at room temperature in a dry place prior to use. Oven-dried (50-60°C) [13] leaves of the plant were divided into three parts weighing 25 g each. Then they were finely powdered and extracted by maceration with (3×250 mL) of water at ambient temperature for 12 h with continuous stirring. The process was repeated thrice. The aqueous extracts were filtrated with No. 1 Whatman filter paper and concentrated to dryness with a rotary evaporator at 50 ± 1°C to give solid residues. The yield was calculated to be 16.71 ± 0.7%. The dried extracts were kept in the dark at 4ºC prior analysis.
Drugs and chemicals
Statin was purchased from Mysore, Solvents like methanol, chloroform, petroleumether, acetone, di-ethyl ether were of analytical grade (Rankem, Bangalore, India). Serum Cholesterol kit, Serum Triglyceride kit, Serum HDL Cholesterol kit, Serum LDL-Cholesterol kit, SGOT, SGPT, ALP were purchased from Aggape (Kumar diagnostic, Mysore). Standard mixtures of fatty acid methyl esters (C8- C24) were procured from Supelco-Sigma Aldrich, Bangalore, India.
Total phenolic contents
Total phenol content was determined using [14] method. One and a half millilitre of Folin–Ciocalteu’s reagent (diluted 10 times) and 1.2 mL of Na2CO3 (7.5% w/v) solution were added to 300 mL of plant extract. Solution was shaken and kept to stand at room temperature (25 ± 2ºC) for 30 min prior to measuring absorbance at 765 nm with a spectrophotometer (Anthelie Advanced 5 Secoman, France). The total phenol content (TPC) was expressed as gallic acid equivalent in mg/100 g fresh plant material.
Determination of antioxidant activity
The capability of a compound to donate a hydrogen atom was assessed on the basis of the scavenging activity of the DPPH (1,1- diphenyl-2-picrylhydrazyl) radical according to Miliauskas et al. [15] with slight modifications. Two millilitres of 0.15 mm DPPH was added to 1 mL of extracts in different dilutions. A control was prepared by adding 2 mL of DPPH to 1 mL of methanol. The contents of the tubes were mixed, allowed to stand for 30 min and absorbance was measured at 517 nm. Triplicate tubes were prepared for each extract. The results were expressed as % radical scavenging activity, which is:
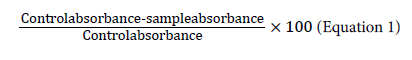
Determination of phenolics by HPLC procedure
The HPLC–MS system was composed of Shimadzu LC20AD HPLC system, ShimadzuSPD20AV UV–vis detector, and Shimadzu 2010EV mass spectrometer fitted with an electrospray interface (Shimadzu, Kyoto, Japan). The column was an Alltech PrevailC18 (Alltech, Deerfield, IL), 150 mm to 4.6 mm. The mobile phase was 0.05% (v/v) aqueous acetic acid (phase A) and 0.05% acetic acid in 80% acetonitrile +20% methanol (phase B) at a flow rate of 0.8 mL/min. The linear gradient of phase B was 5%for the first 2 min, increased from 5% to 40% from 2 min to 48 min, maintained at 40% from 48 min to 57 min, and decreased from 40% to 5% from 57 min to 57.1 min. Finally, isocratic elution with 5% phase B was maintained until 65 min. UV–vis detector wavelength was set at 280 nm. Mass spectra were acquired in negative ion mode. Ion was scanned from m/z 150–600 with scan speed 1000 amu/s. Nebulizing gas flow was 1.5 L/min. Drying gas pressure was 0.1 MPa.
Lipid extraction and analysis of fatty acids
Total lipids were extracted by the method of Bligh and Dyer [16] with minor modifications. In brief, 0.5 g of dehydrated leaf sample was homogenized with chloroform–methanol (2:1 v/v) solution. To the homogenate, 1% KCl (w/v) was added and the content was filtered through filter paper (Whatman no 1). The filtrate was allowed to separate into two phases and the lower (chloroform) phase was collected into previously weighted test tube, dried under the stream of N2 vapour and total lipid content was determined gravimetrically. To find out fatty acid profile of lipid, fatty acid methyl esters (FAME) were prepared using BF3 in methanol method [17]. Peak identification of fatty acids was performed by comparison of the retention time with respective reference standards.
Analysis of FAME by GC-MS
FAMEs were analysed by GC-MS (Perkin Elmer, Turbomass Gold, Mass spectrometer) equipped with FID using fused silica Rtx-2330 column (Restek made, 30 m×0.32 mm ID and 0.20 μm film thickness]. Injector port and the detector were set up at 230 and 250°C respectively. N2 was used as a carrier gas. Initially, column temperature was maintained at 120°C, followed by increasing to 220°C in 20 min, and hold at 220°C for 10 min. The fatty acids were identified by comparing their fragmentation pattern and retention time with authentic standards and also the NIST library.
Animals grouping
Male Wistar rats (OUTB-Wistar) weighing 150 g ± 10 g were grouped by random distribution n=6. They were placed in individual cagesin an approved animal house facility at CSIR-Central Food Technological Research Institute, Mysore, India. Institutional animal ethical committee approved the experimental protocol. Animals were given fresh diet daily and left over dietswere weighed and discarded. The Gain in body weight of animal was monitored at regular intervals. The animals had free access to food and water throughout the study. They were maintained under standard laboratory conditions (temperature 20 ± 3°C; 12 h light–dark cycle).
Induction of hypercholesterolemia with high cholesterol diet
The rats were fed ad libitum AIN-93 [18] supplemented (control group), and other groups were fed ad libitum AIN-93 diet containing with 1% cholesterol (experimental group). The feeding experiment was conducted over aperiod of 60 days. The experimental procedures was carried out according to the National Institute of Health Guidelines for Animal Care and approved by the Ethics Committee of our Institution.
In the present study, four doses of the AEP extract were selected as 50 mg, 100 mg, 150 mg and 200 mg/kg bw.
The rats were randomly divided into seven groups of six each (Figure 1).
Group (Control): The Normal group in which the rats were daily given AIN-93 diet.
Group II: Hyperlipidemic group in which the rats were daily administered with 1% cholesterol in diet.
Group III: Test group in which the rats were daily administered with aqueous extract of purslane (50 mg/kg/day) along with 1% cholesterol in the diet.
Group IV: Test group in which the rats were daily administered with aqueous extract of purslane (100 mg/kg/day) along with 1% cholesterol in diet.
Group V: Test group in which the rats were daily administered with aqueous extract of purslane (150 mg/kg/day) along with 1% cholesterol in the diet.
Group VI: Test group in which the rats were daily administered with aqueous extract of purslane (200 mg/kg/day) along with 1% cholesterol in the diet.
Group VII: Standard group in which the rats were daily administered with Atorvastatin (20 mg/kg/day) along with 1% cholesterol in the diet.
Body weight
The body weight (in g) was recorded on day one and then weekly consecutively for 59 days using a laboratory animal weighing balance.
Biochemical analysis
All the animals were sacrificed on the 60th day of the experiment by cervical dislocation. The blood samples were collected by cardiac puncture and separately sterilized into dry centrifugation tubes and allowed to stand for 30 min at 20-25ºC.
The clear serum was separated at 3000 rpm for 10 min using a centrifuge. Levels of serum glucose, total TC, HDL-C, TG, LDL-C, SGOT, SGPT and ALP were determined to analyze using auto-analyzer (semi-autoanalyzer Erba), with commercial kits (Kumar diagnostics Pvt Ltd.). The Friedewald formula was used to calculate VLDL (=total cholesterol-HDL-LDL) and atherogenic index (=(TCHDL-C)/HDLC)).
In-vivo antioxidant activity: preparation of liver homogenate
The liver samples stored were thawed and used for analysis of antioxidant enzyme. Liver samples were homogenized in ice-cold 0.1 M phosphate buffered saline of pH 7.4 (10% homogenate) followed by centrifugation at 10,000 rpm for 10 min. The supernatant was used to prepare aliquots of homogenates to carry out the superoxide dismutase (SOD) and catalase assays.
Estimation of super oxide dismutase (SOD)
An indirect method of inhibiting auto-oxidation of epinephrine to its adrenochrome was used to analyze SOD activities in blood serum. About 0.5 mL of blood serum was diluted with 0.5 mL of distilled water, to this 0.25 mL of ethanol; 0.5 mL of chloroform (all chilled reagents) was added. The mixture was allowed to vortex for 1 min and centrifuge at 2000 rpm for 20 min.
The SOD activity in the supernatant was determined by the addition of 0.05 mL of carbonate buffer (0.05 M pH 10.2) and 0.5 mL of EDTA. The reaction was initiated by addition of 0.4 mL of epinephrine and read the O.D using spectrophotometer at 480 nm. The SOD activity was expressed as Units/mg protein transformed in optical density/mm. About 50% inhibition of epinephrine to adrenochrome transition by the enzyme is taken [19].
Estimation of catalase (CAT)
The activity of catalase (CAT) in liver homogenate was measured by the decrease in absorption at 240 nm using spectrophotometer. Reaction mixture consists of 995 microliter phosphate buffer (0.1 nm, ph-7), 5 mL of sample and 50 mL of (8.8 nM). One CAT activity is defined as the amount of enzyme required to decompose I milt of H2O2/min liver homogenate [20].
Statistical analysis
Results were presented as mean ± S.D. The significance of variation among the groups was observed using one way analysis of variance (ANOVA). All the experiments were repeated thrice.
Hyperlipidemia continues to be a main health problem in India and other developing countries, which lead to vital risk factors like atherosclerosis, stroke etc.
Hyperlipidemia evokes the damages in various tissues, which in turn, deregulates the cellular functions foremost to various pathological conditions. It is known that nutrition plays an important role in the etiology of hyperlipidemia and atherosclerosis [21].
Phytochemical analysis
The results of the preliminary phytochemical screening of aqueous extract of purslane leaves (AEP) showed the presence of total polyphenol, antiradical activity (Table 1a) and flavonoids (Table 1b).
| Total Polyphenol content(mg GAE/100DW) | 283.17 ± 0.6 |
| Antiradical activity (%) | 86.79 ± 0.14 |
| Values are expressed as mean ± standard deviation | |
Table 1a: Total polyphenol content and antiradical activity of aqueous extract of dried purslane.
| Sample | Phenolic acids (mg/100g dry wt.) |
Flavonoids content (mg/100g dry wt.) |
||||
|---|---|---|---|---|---|---|
| Rosmarinic acid | Ferulic acid | Kaempferol | Quercetin | Coumaric acid | ||
| Aqueous extract of Purslane | 0.55 ± 0.02 | 4.85 ± 0.06 | 0.41 ± 0.08 | 0.92 ± 0.03 | 0.34 ± 0.02 | |
| Values are expressed as mean ± standard deviation | ||||||
Table 1b: Phenolic acids and flavonoids content of aqueous extract of dried purslane.
Fatty acid profile of purslane
The Tables 2a and 2b showed that the presence of PUFA and α- linolenic acid (ALA) was observed in the dried purslane to an extent of 52.65% and 60.97% respectively.
| Capric | Lauric | Myristic acid | Palmitic | Stearic acid (C18:0) | Oleicacid (C18:1) | Linoleic acid (C18:2) | α-Linolenic acid |
|---|---|---|---|---|---|---|---|
| acid | acid (C12:0) | (C14:0) | acid | (C18:3) | |||
| (C10:0) | (C16:0) | ||||||
| 1.4 ± 0.2 | 0.75 ± 0.1 | 0.8 ± 0.3 | 19.7 ± 0.1 | 7.1 ± 0.3 | 9.5 ± 0.2 | 12.1 ± 0.1 | 48.6 ± 0.3 |
Table 2a: Fatty acid (% relative area).
| SFA | MUFA | PUFA | ALA |
|---|---|---|---|
| 29.75 ± 0.2 | 9.5 ± 0.1 | 60.7 ± 0.2 | 48.6 ± 0.3 |
| Where, S: Saturated; MU:Monounsaturated; PU: Polyunsaturated; FA: Fatty acids; ALA: α-Linolenic acid | |||
Table 2b: Distribution of SFA, MUFA and PUFA content of dried purslane.
Effects of high-fat diet and AEP on body and organ weight
There was no difference among the treatment groups at the commencement. However upon animals fed with cholesterol diet showed significant increase in body weight compared to those feed with control diet (p<0.01) (Table 3). The untreated hyperlipidemic group showed significantly higher organ weight (liver) in comparison with HCD group (p<0.05). However, no significant difference was found in the weight of kidney and heart between high-fat diet and control group.
| Initial weeks | Control (g) | Untreated(g) | Drug Treated(g) | 50 concentration (mg/kgbw) | 100 concentration (mg/kgbw) | 150 concentration (mg/kgbw) | 200 concentration (mg/kgbw) |
|---|---|---|---|---|---|---|---|
| Initial bw(g) | 193.4 ± 1.3 | 180 ± 2.1 | 198 ± 0.9 | 195.75 ± 1.4 | 201.25 ± 2.3 | 195 ± 1.4 | 200.5 ± 1.3 |
| 2nd | 224.0 ± 0.7 | 231.0 ± 2 | 233.0 ± 0.7 | 235.0 ± 0.8 | 213.0 ± 1.4 | 230.0 ± 0.8 | 239.1 ± 2.6 |
| 3rd | 230.0 ± 0.9 | 247.0 ± 1 | 240.0 ± 0.8 | 244.0 ± 0.6 | 225.0 ± 1.7 | 235.0 ± 2.5 | 226.0 ± 1.9 |
| 4th | 250.0 ± 0.6 | 288.0 ± 1 | 261.7 ± 1.3 | 259.7 ± 1.5 | 254.5 ± 1 | 260.0 ± 1.6 | 252.2 ± 0.9 |
| 5th | 262.5 ± 1.4 | 316.0 ± 2 | 271.2 ± 0.8 | 271.2 ± 1.7 | 266.0 ± 0.6 | 278.5 ± 1.3 | 268.7 ± 0.5 |
| 6th | 269.2 ± 0.9 | 330.0 ± 0.6 | 278.0 ± 2.4 | 281.2 ± 1.2 | 272.4 ± 1 | 285.2 ± 0.7 | 285.2 ± 1.4 |
| 7th | 278.5 ± 2.4 | 341.0 ± 2 | 296.6 ± 1.7 | 289.7 ± 1.8 | 281.2 ± 1.3 | 296.2 ± 0.5 | 289.2 ± 2.3 |
| Final bw | 265.0 ± 1.6 | 333.7 ± 0.5 | 285.0 ± 1.4 | 273.5 ± 1.4 | 267.0 ± 0.8 | 288.0 ± 1.6 | 280.0 ± 1.2 |
| Liver weight | 7.75 ± 0.3 | 9.8 ± 0.6 | 7.8 ± 0.5 | 7.95 ± 0.4 | 7.46 ± 0.3 | 7.65 ± 0.6 | 7.47 ± 0.7 |
| Heart weight | 0.8 ± 0.5 | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.80 ± 0.7 | 0.75 ± 0.2 | 0.75 ± 0.4 | 0.53 ± 0.3 |
| Kidney weight (g) | 1.32 ± 0.9 | 1.8 ± 0.7 | 1.63 ± 0.4 | 1.5 ± 0.6 | 1.34 ± 0.7 | 1.57 ± 0.7 | 1.65 ± 0.6 |
| Where, bw:Body weight of animals | |||||||
Table 3: Variation in body weight of rats fed a 1% cholesterol diet and treated with AEP extracts for 7 weeks.
Effect of administration of AEP on biochemical parameters of high cholesterol diet induced hyperlipidemia
The effect of AEP on lipid levels of serum in treated rats is shown in Figure 2. The results showed that the levels of TG, TC, LDL-C and VLDL-C in the HCD treated group were significantly higher (p<0.05), while the level of HDL-C was significantly lower (p<0.01), when compared to normal and other treated groups. The results on serum lipid profile of HCD groups with doses of AEP (150 mg/kg), showed significant (p<0.01) decrease in serum parameters TG, TC, LDL, VLDL and significantly (p<0.05) increase in HDL, whereas atorvastatin showed significantly (p<0.05) decrease in TG, TC, LDL, VLDL and increase (p<0.01) in HDL when compare with that of high cholesterol diet group. The effect of AEP as a feed supplement at four doses, i.e., 50, 100, 150 and 200 mg/kg bw, resulted in a dosedependent reduction in lipid profiles.
Excessive amounts of PUFA (omega6 fatty acids) and a very high omega6 fatty acids/omega3 fatty acids ratio, as is found in today's Western diets, promote the pathogenesis of many diseases, including cardiovascular disease, cancer, inflammatory and autoimmune diseases, whereas increased levels of omega3 fatty acids (a lower omega- 6 fatty acids/omega3 fatty acids ratio), exert suppressive effects. In the secondary prevention of cardiovascular disease, a ratio of 4/1 was associated with a 70% decrease in total mortality. In our study also we found that 200 mg/kg wt group rats were having some toxic effect.
Effects of AEP on LDL/HDL and Atherogenic index
As seen from Figure 2 the leaves of LDL/HDL and Atherogenic index are higher in the HCD treated groups compared to normal and other treated groups.
Effect of AEP extract on liver enzymes
As shown in Table 4a, treatment with AEP extracts exhibit a hepatoprotective effect, indicated by decreased levels of SGOT, SGPT, and ALP. The increased liver enzyme levels and the formation of hepatic steatosis (fatty liver) in the high cholesterol diet-fed groups are allied with a significant increase of liver weight. The administration of the extract resulted in the prevention of hepatic fatty deposition in hepatocytes.
| Control | Untreated | Drug Treated | 50 concentration (mg/kgbw) | 100concentration (mg/kgbw) | 150 concentration (mg/kgbw) | 200 concentration (mg/kgbw) | |
|---|---|---|---|---|---|---|---|
| SGOT U/dL | 40.14 ± 0.4 | 56.71 ± 1.2 | 38.83 ± 0.9 | 58.02 ± 1.4 | 41.88 ± 0.6 | 47.99 ± 1.1 | 46.05 ± 0.8 |
| SGPT U/dL | 56.78 ± 0.3 | 78.17 ± 1.4 | 54.15 ± 0.9 | 58.75 ± 1.5 | 64.56 ± 0.7 | 40.57 ± 1.4 | 57.94 ± 1.6 |
| ALP U/dL | 152 ± 0.7 | 256 ± 1.1 | 157 ± 0.8 | 280 ± 1.4 | 211 ± 1.6 | 172.4 ± 0.8 | 225 ± 1.8 |
| Values are expressed as mean ± SE of animals, p values <0.05 | |||||||
Table 4a: Effect of AEP on liver enzyme activity in normal and hypercholesterolemic rats.
The superoxide dismutase (SOD) activity in the hyperlipidemic control group has shown (Table 4b) considerable decline as compared to the normal group. The treatment with standard drug Atorvastatin as well as the four doses of the AEP led to the significant (p<0.05) increase in SOD activity levels thus demonstrating the antioxidant activity of the extract. The Atorvastatin treated group showed the highest increase in SOD activity, followed by the 200 mg/kg body weight. As it is seen from Table 4b, the catalase activity has also showed a considerable decrease in the hyperlipidemic control group. Treatment by the standard drug Atorvastatin and the two doses of the AEP led to a significant (p<0.05) increase in catalase activity. The Atorvastatin treated group showed the highest increase in catalase activity, followed by the 150 mg/kg body weight doses of the AEP. The cholesterol lowering effect of purslane may be attributed to the combined effect of omega-3 fatty acids, phenolics compound and pectin [22]. As shown in the present study, while elevating plasma HDL-c levels, AEP lowered to plasma total cholesterol and LDL-c levels dose dependently. There is a positive correlation exists among the risk of developing ischemic heart disease and raised plasma total cholesterol and LDL-c concentrations [23]. The weight losing effect of AEP observed in this study could either be as a result of its lipid lowering or appetite inhibiting of binding affinity of pectin with dietary lipids that is not available for absorption [24]. The protective role of glutathione, as an antioxidant and detoxifying agent, has been demonstrated in various clinical studies [25]. It is a ubiquitous compound that is synthesized rapidly in the liver, kidney and other tissues, including the gastrointestinal tract. In animal cells, glutathione acts as a substrate for glutathione peroxidase, which reduces lipid peroxides that are formed from polyunsaturated fatty acids (PUFA) in the diet and as a substrate for glutathione S-transferase, which conjugates electrophilic compounds. Many evidences showed that glutathione obtained from the diet is directly absorbed by the gastrointestinal tract and thus dietary glutathione can readily increase the antioxidant status in humans [26]. The antioxidant enzymes such as GPx, GR, SODand GST, take part in maintaining GSH homeostasis in tissues [27]. Also, increased levels of GPx, GR, GST, CAT and SOD, were all found to correlate with elevated glutathione level and depressed MDA and NO in rats, showing the antioxidant activity of purslane. In conclusion, purslane leaf is beneficial for hepatic and cardio-protective activities as it has an antioxidant properties and nutritive value.
| Dosage Groups | SOD | Catalase |
|---|---|---|
| (Units/min/mg protein) | (μmoles of H2O2/min/mg) | |
| Control | 12.33 ± 0.5 | 222.5 ± 2.8 |
| High Cholesterol Die (HCD) | 5.31 ± 0.2 | 110.3 ± 2.6 |
| HCD+AEP 50 mg/kg | 6.18 ± 0.2 | 132.7 ± 1.1 |
| HCD+AEP 100 mg/kg | 8.12 ± 0.4 | 149.8 ± 3.2 |
| HCD+AAEE 150 mg/kg | 10.22 ± 0.4 | 192.8 ± 2.2 |
| HCD+AEP 200 mg/kg | 9.12 ± 0.3 | 205.8 ± 1.4 |
| HCD+STD (Atorvastatin) | 11.08 ± 0.8 | 237.5 ± 3.6 |
| Values are expressed as Mean ± SEM, (n=6). Significant difference compared to control; significant difference compared to HFD, P<0.05 | ||
Table 4b: Effect of AEP on antioxidant activity of SOD and catalase in hyperlipidemic rats.
Purslane is used traditionally in medicine to manage, control, and/or treat diabetes mellitus and hypertension. The present study demonstrate the aqueous extract (50-200 mg/kg bw) of leaves fed to hyperglycaemic rats indicate that the leaf aqueous extract of purslane possesses hypolipidemic properties, and thus purslane can be used in the management or control of hyperlipidemia. However, dietary studies are needed to further confirm the impact of purslane on lipid metabolism. Further purslane can be used as an easily accessible source of natural antioxidants.
The authors thank Professor Ram Rajasekharan, Director, CSIRCFTRI, Mysore, for providing infrastructure, constant encouragement and support. The authors also thank the Head, Department of Animal House, CSIR-CFTRI, Mysore, for providing all the facilities and suggestions during animal study. The first author gratefully acknowledges U.G.C for providing the financial assistance to carry out the present investigation. The help of Dr V Baskaran, Senior Principal Scientist, Department of Biochemistry, CSIR-CFTRI, Mysore, is highly acknowledged.