Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2022)Volume 13, Issue 5
The African sharp tooth catfish (Clarias gariepinus) is the most important catfish species for aquaculture. It has a great capacity to endure several stressing factors, such as adverse environmental conditions, besides having broad feeding flexibility. Notwithstanding, the presence of this alien fish species in the Guapimirim Environmental Protection Area, in southeast Brazil, may have negative impacts on the community of native fish. In 2018, during dry and rainy seasons, samples from the fish community were collected in 32 sites of the Guapi-Macacu River, in addition to abiotic variables (salinity, pH, temperature, turbidity, dissolved oxygen, and transparency) to diagnose which factors influence the distribution of the alien fish along the river. Moreover, species were evaluated as bio indicators in the ichthyofauna to identify potential alterations in the community. Multivariate analyses indicated that the African sharp tooth catfish dominates the buffer zone of the environmental protection area, benefiting from higher levels of dissolved oxygen and temperature. However, C. gariepinus still does not dominate the most protected area of Guapimirim, where a higher percentage of native fish species dwell. Alterations in abiotic factors, related to the increase in the temperature, can significantly contribute to the dominance of this invasive alien fish in this protected area, requiring constant monitoring of some key species, as well as the population of the invasive species in this environmental preservation area.
Invasive alien fish; Overlapping habitats; Abiotic variables; Bioindicators; Climate change
Invasive Alien Species (IAS) is considered one of the main threats to biodiversity and an important component of global environmental change [1]. Once present and acclimated, the IAS becomes difficult to control and results in marked changes in the native fauna, compromising the balance and stability of the ecosystem by reducing their stocks and causing extinctions, competition, pathogen transmission, and hybridization [1-5].
The African sharp tooth catfish–Clarias gariepinus is a native species to much of the African continent and parts of southwest Asia, such as Israel, Syria, and southern Turkey [6,7]. It has the exceptional ability to migrate to the terrestrial environment and breathe atmospheric air through pseudolungs, which allows it to endure adverse conditions of temperatures and low oxygen concentrations [7]. Clarias gariepinus was introduced in Brazil in the 1980s for aquaculture and fish-and-pay lakes [8]. The escape of the African catfish from aquaculture systems to the natural environment has threatened several species of native fish, making it a potent IAS for these ecosystems [9]. The potential invasions of C. gariepinus have shown that the species can progressively establish itself depending on the source of the invader's dispersion, causing a reduction in food availability and pressure on the ecosystem’s trophic chain [10-12]. Added to this factor, the invasiveness of the African catfish can happen in a short period, resulting in malnutrition, low growth, or even elimination of some native species [13]. In other cases, unplanned hybridization, with an escape into the natural environment, can cause the irreversible loss of native biodiversity [14].
The Guapimirim Environmental Protection Area (Guapimirim APA) is located northeast of the Guanabara Bay, one of the most environmentally critical coastal areas of the Brazilian coastline, in Rio de Janeiro, Brazil. The Guapimirim APA is a Federal Conservation unit, created in 1984 to protect the remaining mangrove and to ensure the survival of human populations that still depend on this environment. The Guanabara Ecological Station (Guanabara ESEC) is located within the APA. The ESEC is a fully protected Conservation Unit, with no admittance of people within its borders, except for scientific or educational purposes. The Guapimirim APA has several rivers and canals; among them, the Guapi-Macacu River stands out, due to its water output, supplying drinking water to most municipalities in its eastern portion [15]. In this river, in particular, the riverine population is aware of the presence of the African catfish; however, we know little about its distribution and its possible impacts on the native ichthyofauna of this area.
In this regard, the riverine population must receive detailed information on the distribution of invasive species, so that they can detect potential changes in the biotic community of aquatic ecosystems, considering the fish community as a whole, and which abiotic or environmental factors may be involved in the establishment of exotic species. In Brazil, some studies report the occurrence of C. gariepinus in natural environments and environmental protection areas [16,17]. Weyl et al., who conducted an extensive review of studies on the establishment of alien populations of African sharp tooth catfish, mention that few biological assessments are available, especially studies focused on their persistence and long-term establishment using empirical ecological data [18]. For these authors, establishment is the most important phase of the invasion process, as negative impacts are more likely to occur when alien species become established.
To determine the integrity of ecosystems, the characterization of physicochemical parameters is not sufficient; and the scarcity of methods to objectively incorporate the degradation of the biological component also implies a lack of responses to the increasingly negative effects of IAS [19,20]. An alternative to obtaining a response regarding the biotic integrity is to look for “bio indicators”, whose presence and abundance reflect changes that occur throughout the community or at the ecosystem level [21-23].
Therefore, to assess the integrity of the fish community and the establishment of this IAS in this protection area it is necessary to first assess the fish community itself and the main abiotic components for its structure. In this sense, this study aims to characterize the environmental conditions favorable to the alien species, by contemplating the physicochemical parameters of the water, as well as inferring possible bio indicator species of bio ecological impacts on the fish community. To accomplish this, it is essential to characterize the distribution of the African catfish population and the native fish community along the Guapi-Macacu River.
Area of study
The physiography of the Guapi-Macacu River is divided into three segments or areas. The first flows down the slope of the Serra do Mar in the form of rapids and waterfalls, with its banks covered with Atlantic Forest. A second area runs through a transition between the cliffs and the plains, with a less rugged formation. In this stretch, the river is surrounded by riparian forest and hills with rounded, massive shapes with altitudes below 1000 m. And a third area, which is longer and runs through a floodplain, with flat terrain and minimal unevenness, is easily flooded and subject to tidal influences due to its proximity to the Guanabara Bay. The vegetation of this segment is composed of small shrubs, pastures, and mangroves and is closer to the most significant urban centers [24-26].
Sampling
Community sampling was conducted in February (rainy season) and August 2018 (dry season) in the Guapi-Macacu river using nets with different meshes, fishing traps, fyke nets, hooks, and lines. The fishing gear was installed and used at night, as the invasive species, the target of the study, is nocturnal. At the same time, a Hanna multiparameter probe (model HI 9828) was used to measure in situ the physicochemical variables of the water: temperature, pH, dissolved oxygen (mg/L); transparency (cm), turbidity (FNU); and salinity (PSU). Sampling was distributed along 32 points: 10 points between the mouth of the river and the inner limit of the Guanabara ESEC (downstream area); 12 points between the external boundary of the Guanabara ESEC and the internal perimeter of the Guapimirim APA (intermediate zone); and 10 points distributed between the outer limit of the Guapimirim APA and the dam (upstream area), within the Buffer Zone of the Guanabara Ecological Station (Guanabara BZ), totalling 64 sampling points in both seasons (Figure 1). Gillnets were removed 24 hours after installation. The fish captured were grouped at each sampling point and packed in plastic bags, labeled, and refrigerated on ice, and then transferred to the Laboratory of Applied Ecology at UFF.
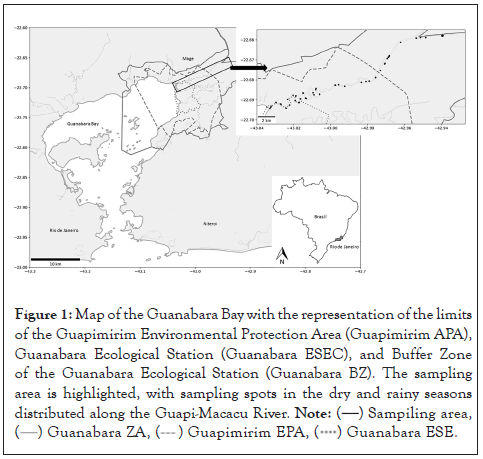
Figure 1: Map of the Guanabara Bay with the representation of the limits of the Guapimirim Environmental Protection Area (Guapimirim APA), Guanabara Ecological Station (Guanabara ESEC), and Buffer Zone of the Guanabara Ecological Station (Guanabara BZ). The sampling area is highlighted, with sampling spots in the dry and rainy seasons distributed along the Guapi-Macacu River.
Laboratory activities, data processing, and statistical analysis
The collected fish were identified, weighed, and measured. To characterize the fish communities, the following indices were assessed: species richness, abundance, biomass, diversity, dominance, and equitability. Richness was calculated using the total number of species collected at each sampled point, as well as abundance. For the analysis of diversity, the Shannon-Wiener index, the Simpson dominance index, and the Pielou uniformity index were used [27].
The Permutational Multivariate Analysis of Variance (PERMANOVA) was used in the R Program (R Core Team, 2020), available in the VEGAN package, to test for spatial and seasonal differences in the physicochemical variables of water and ecological descriptors of the ichthyofauna [28]. PERMANOVA was also applied to test the spatial and temporal variation of species richness, abundance, and fish biomass, in addition to the indices of diversity, equitability, and dominance. PERMANOVA is similar to the traditional ANOVA, although it does not require the assumptions of normality and homoscedasticity [29-31]. The Bray-Curtis distance was used in all PERMANOVA tests, permuted 4999 times per analysis, as recommended by Manly.
The Multinomial Species Classification Method (CLAM) was used, through the "CLAMTEST" function available in the VEGAN package, to classify the species into generalists or specialists during rainy and dry seasons without excluding rare species [32,33]. This method uses a multinomial model to estimate the relative abundance of species in two groups (A, B), minimizing adverse effects due to differences or insufficiency of sampling within each habitat [34]. A threshold of 50% specialization in each period was set, with a 95% significance level for individual tests.
To analyze the spatial proximity of the species and the formation of groups, according to the sampling points in both periods (considering the river as a whole), Cluster Analysis was used, through the "HCLUST" function of the package DENDEXTEND, using the "ward. D2" with the Bray-Curtis distance in the dissimilarity matrix of the VEGAN package [35-37]. The sum of squares criterion was used for this agglomerative method, producing groups that minimize the dispersion within the group in each binary merger [38]. This analysis was used to infer the similarity in the distribution of species in the river stretches, according to their abundance.
As a way of assessing potential species as bioindicators of the fish community, the specificity and fidelity analysis (IndVal) was used, which performs a permutation test (with a significance of 0.05). For this, the "MULTIPATT" function, available in the INDICSPECIES package, was used both to determine the indicator species between seasons (rainy and dry) and river areas and between river segments, regardless of the season [39]. The IndVal method has some advantages when compared to other bioindication methods; it is calculated for each species independently, where the categorization of habitats occurs without restrictions, and may be grouped subjectively or quantitatively [40].
The Canonical Correspondence Analysis (CCA), which is considered one of the best methods for the analysis of direct gradient in community ecology, was used in the R program version 4.0.2 (R Core Team, 2020), available in the VEGAN package, and applied to the environmental and biological data matrix (excluding the rare species previously selected in the CLAM model) [41,42]. It was used to identify which physicochemical variables of the water contributed the most to the characterization of the river points in the rainy and dry seasons, as well as to verify the distribution of the abundances of the fish species found, correlating them with the environmental variables (complete model). The multicollinearity of the model was diagnosed through the "ORDISTEP" function, which performs an automatic selection, based on the permutation test using the P-value. The variable selection procedure based on the P-value aims to find the ideal model, in which only the most significant environmental variables explain the model [43]. Subsequently, the significance of both models (full model and reduced model) was tested, as well as which axes and terms were significant, by Analysis of Variance (ANOVA), using the "ANOVA. CCA" function. Therefore, an ideal and reduced model for the Guapi-Macacu River was obtained, displaying the environmental variables that can predict changes in the composition and abundance of the ichthyofauna and indicate which species will be affected in this relationship.
PERMANOVA identified significant differences for all environmental variables between the areas and seasons studied, except for temperature, which did not exhibit evident differences between the river segments (Table 1). In general, water temperature was higher in the rainy season. Salinity was higher downstream in the dry season, but much lower in the rest of the groups (location and season combined). Transparency was higher in the dry season upstream. Dissolved Oxygen (DO) was higher in the dry season than in the rainy season. Higher DO values occurred in the upstream segment during the dry season, followed by the intermediate and downstream areas. In the rainy season, DO was higher in the upstream region, followed by the intermediate and downstream regions. The pH was also higher in the dry season than in the rainy season. Higher values were recorded in the upstream segment during the dry season, followed by the downstream and intermediate zones. During the rainy season, the pH had similar values in three stretches of the river. The highest turbidity values occurred in the rainy season downstream, followed by intermediate and low segments. During the rainy season, turbidity did not vary significantly between the three areas of the river (Figure 2).
| Parameter | Area | Season | ||
|---|---|---|---|---|
| F | P | F | P | |
| Salinity | 19.93 | <0.001 | 11.799 | <0.001 |
| Temperature | 0.4311 | 0.66 | 299.6786 | 0.0002 |
| pH | 4.2159 | 0.02 | 92.8138 | 0.0002 |
| DO | 5.1768 | 0.008 | 27.1637 | 0.0002 |
| Transparency | 15.038 | <0.001 | 189.762 | <0.001 |
| Turbidity | 17.625 | <0.001 | 102.902 | <0.001 |
Table 1: PERMANOVA analyses of environmental variables in the Guapi-Macacu River with segments and seasons (dry/rainy) as factors.
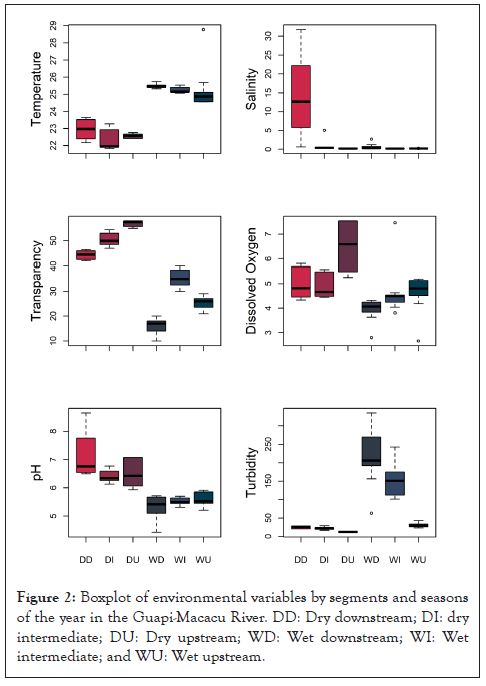
Figure 2: Boxplot of environmental variables by segments and seasons of the year in the Guapi-Macacu River. DD: Dry downstream; DI: dry intermediate; DU: Dry upstream; WD: Wet downstream; WI: Wet intermediate; and WU: Wet upstream.
Thirty-one species were collected, with a total abundance of 428 specimens in terms of ichthyofauna, distributed in nine orders and 21 families (Table 2). The distribution of the relative abundance of the fish community along the Guapi-Macacu River between the areas and seasons sampled is shown in Figure 3. PERMANOVA detected significant differences for fish abundance in relation to the area (F=2.6214; p=0.0454) and periods (F=6.9902; p=0.0020). The highest abundance of medians occurred in the dry season downstream.
| Species | Code | Abundance | Biomass (g) | Size class (mm) | Classification (CLAM) |
|---|---|---|---|---|---|
| Acestrorhynchus lacustres | ALA | 4 | 261.068 | 130-257 | Rare |
| Astyanax altiparanae | AAL | 7 | 80.16 | 90-100 | Rare |
| Astyanax fasciatus | AFA | 1 | 15.867 | 115 | Rare |
| Brevoortia pectinata | BPE | 15 | 1000.301 | 143-300 | Specialist/Dry |
| Centropomus parallelus Poey | CPA | 28 | 5563.947 | 120-415 | Specialist/Rainy |
| Centropomus undecimalis | CUN | 14 | 3365.919 | 125-455 | Generalist |
| Clarias gariepinus | CGA | 30 | 43970 | 345-830 | Generalist |
| Cynoscion acoupa | CAC | 1 | 372 | 350 | Rare |
| Cyphocharax gilbert | CGI | 7 | 986.376 | 178-208 | Rare |
| Diapterus rhombeus | DRH | 7 | 243.309 | 105-159 | Rare |
| Elops saurus Linnaeus | ESA | 8 | 758.023 | 201-293 | Rare |
| Eucinostomus argenteus, Bair and Girard | EAR | 4 | 274.482 | 153-195 | Rare |
| Eugerres brasilianus | EBR | 30 | 2794.393 | 114-370 | Specialist/Dry |
| Genidens genidens | GGE | 57 | 5163.797 | 105-320 | Generalist |
| Geophagus brasiliensis | GBR | 8 | 1336.527 | 78-250 | Rare |
| Gymnotus carapo Linnaeus | GCA | 1 | 272 | 390 | Rare |
| Hoplias malabaricus | HMA | 10 | 5576 | 280-395 | Rare |
| Hoplosternum littorale | HLI | 22 | 1528.875 | 86-190 | Specialist/Rainy |
| Hypostomus auroguttatus Kner | HAU | 51 | 7647.396 | 180-334 | Generalist |
| Leporinus friderici | LFR | 1 | 242 | 264 | Rare |
| Loricariichthys castaneus | LCA | 30 | 4379.137 | 259-354 | Generalist |
| Micropogonias furnieri | MFU | 33 | 4003.924 | 110-335 | Specialist/Dry |
| Mugil curema Valenciennes | MCU | 1 | 148 | 250 | Rare |
| Mugil liza Valenciennes | MLI | 3 | 2043.7 | 230-425 | Rare |
| Oligosarcus hepsetus | OHE | 6 | 414.461 | 128-223 | Rare |
| Oreochromis niloticus | ONI | 16 | 3108.394 | 126-362 | Specialist/Rainy |
| Plagioscion squamosissimus | PSQ | 1 | 630 | 330 | Rare |
| Prochilodus lineatus | PLI | 1 | 13.461 | 110 | Rare |
| Rhamdia quelen | RQU | 11 | 2889.96 | 119-375 | Generalist |
| Trachelyopterus striatulus | TST | 18 | 2496.304 | 154-225 | Generalist |
| Trinectes microphthalmus | TMI | 2 | 14.468 | 50-55 | Rare |
Table 2: List of species, code in the analyses, abundance, biomass, size classes, and CLAM classification of the ichthyofauna collected in 2018 along the Guapi-Macacu river.
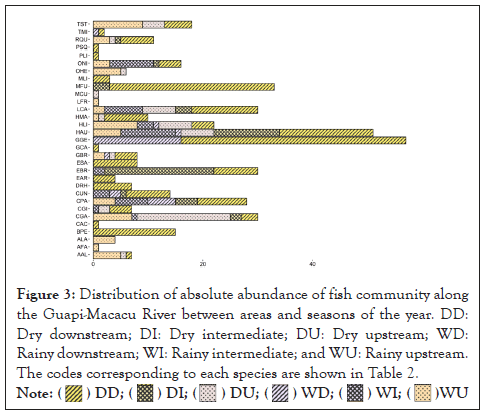
Figure 3: Distribution of absolute abundance of fish community along the Guapi-Macacu River between areas and seasons of the year. DD: Dry downstream; DI: Dry intermediate; DU: Dry upstream; WD: Rainy downstream; WI: Rainy intermediate; and WU: Rainy upstream. The codes corresponding to each species are shown in Table 2.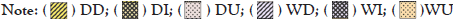
For species richness, PERMANOVA showed significant differences only between the dry and rainy seasons (F=4.8069; p=0.0226). This index indicated the highest medians in the dry season downstream and the lowest median in the rainy season downstream. Biomass had significant differences in PERMANOVA only between the dry and rainy seasons (F=5.8107; p=0.0032). The dominance had differences among the river segments, detected in the PERMANOVA analysis (F=3.6422; p=0.0326). PERMANOVA did not show significant differences between the other zones and seasons, as well as for diversity and equitability (p>0.05, for all) (Figure 4).
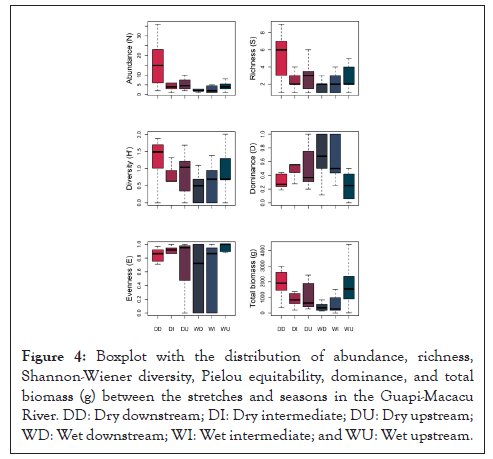
Figure 4: Boxplot with the distribution of abundance, richness, Shannon-Wiener diversity, Pielou equitability, dominance, and total biomass (g) between the stretches and seasons in the Guapi-Macacu River. DD: Dry downstream; DI: Dry intermediate; DU: Dry upstream; WD: Wet downstream; WI: Wet intermediate; and WU: Wet upstream.
The CLAM classification indicated that 22.6% of the species collected were generalists in the dry and rainy seasons, with frequent occurrence in the two seasons studied, while 9.7% were classified as specialists in the rainy season and 9.7% in the dry season. Rare species in the samples corresponded to 58.1% (Figure 5).
Figure 5: CLAM classification of the ichthyofauna in the dry and rainy seasons (p=0.05). Lines represent the specialization limit. Dashed line=rainy season; dotted line=dry season; solid line=rare and abundant species. The codes corresponding to each species are shown in Table 2.
The cluster analysis results coincided with the previous locations shown in Figure 1, representing the physiography of the Guapimirim APA in the spatial separation of the ichthyofauna (Figure 6). The main species that make up the Guapimirim APA were located mainly to the left of the dendrogram. The species on the right are distributed along the upstream area and cover the entire river. The last two groups were formed by rare species. In this classification, the target species C. gariepinus is close to groups with broad distribution in the river, such as L. castaneus, T. striatulus, and H. auroguttatus. Furthermore, C. gariepinus occurs similarly to R. quelen and T. striatulus, sharing the same habitats.
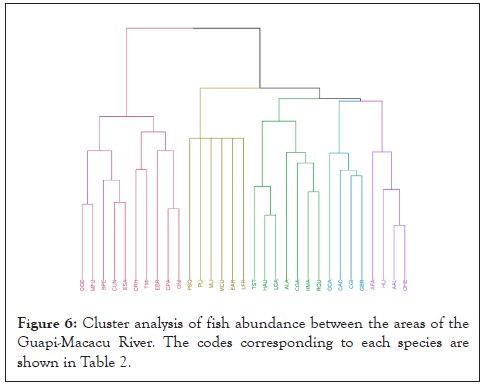
Figure 6: Cluster analysis of fish abundance between the areas of the Guapi-Macacu River. The codes corresponding to each species are shown in Table 2.
The specificity and fidelity analysis (IndVal) showed as indicators the most important species that make up the two main groups formed in the cluster analysis (red line and brown line). As for the specificity and fidelity between areas and seasons, the analysis selected six species. In the dry season downstream, the selected species were B. pectinata (67.9%; p=0.001), M. furnieri (58.0%; p=0.008), and E. saurus (48.0%; p=0.021). Genidens genidens had high fidelity and specificity in the downstream segment in the two seasons sampled (76.9%, p=0.001). Acestrorhynchus lacustres was selected as an indicator species in the upstream segment in the rainy season (52.2%; p=0.008), while T. striatulus was selected in the same segment in the two seasons sampled (53.4%, p=0.035). When the IndVal analysis was performed only for the river segments, seven species were selected and grouped in the two analyzed seasons. In the group formed only upstream, the C. gariepinus (54.8%, p=0.013), T. striatulus (52.6%, p=0.007), and A. lacustres (39.7%; p=0.025) showed fidelity and specificity. In the downstream segment, an exclusive area of the Guanabara ESEC, the function selected G. genidens (76.9%, p=0.001), B. pectinata (52.2%, p=0.003), and M. furnieri (45.2%, p=0.043). C. undecimalis (p=0.022) was the only species selected for both regions of the Guapimirim APA (downstream and intermediate area), demonstrating 51.2% of specificity and fidelity.
The CCA ordination diagram defined 40% (ANOVA, F=1.956; p=0.001) of the total distribution of the species abundance (generalists and specialists selected in the CLAM model) with abiotic variables on the river segments and in the rainy and dry seasons (Figure 7A). The complete model (Figure 7A) displayed the distribution of species with all abiotic variables, with the first axis defining 39.60% of the samples (ANOVA, F=4.9758; p=0.003) and the second axis defining 22.58 % of the samples (ANOVA, F=2.8375; p=0.111), with a relation between the differences in the distribution of fish species. Among the selected environmental variables, ANOVA exhibited significant differences for dissolved oxygen (F=2.2514; p=0.033), transparency (F=2.0854; p=0.028), temperature (F=1.6246; p=0.057), and pH (F=3.7788; p=0.017), which were related to the distribution and abundance of fish species, as well as to the river segments. ANOVA showed no significant differences in CCA for turbidity (F=1.9987; p=0.061) and salinity (F=1.0788; p=0.372).
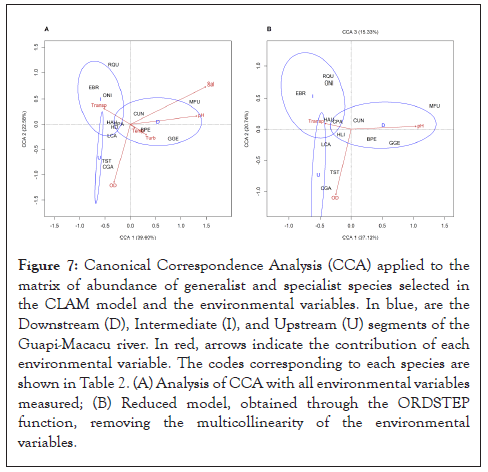
Figure 7: Canonical Correspondence Analysis (CCA) applied to the matrix of abundance of generalist and specialist species selected in the CLAM model and the environmental variables. In blue, are the Downstream (D), Intermediate (I), and Upstream (U) segments of the Guapi-Macacu river. In red, arrows indicate the contribution of each environmental variable. The codes corresponding to each species are shown in Table 2. (A) Analysis of CCA with all environmental variables measured; (B) Reduced model, obtained through the ORDSTEP function, removing the multicollinearity of the environmental variables.
On the other hand, the significance of the axes is altered in the reduced model, which was obtained through the "ORDSTEP" function (Figure 7B). In the second model, the three axes are significant (p<0.02, to all), together explaining 73.19% of the samples. The most significant variables for each analyzed area of the river were distributed as follows: pH had a significant correlation with the species distributed further downstream of the river (F=3.3683; p=0.010); transparency was mainly correlated with species in the intermediate area (F=3.1519; p=0.001); and DO was the most important physicochemical parameter for the species upstream of the river (F=2.5752; p=0.004), all showing high statistical significance.
Abiotic variables in the Guapi-Macacu river
In the Guapi-Macacu River, salinity was higher downstream in both seasons, while DO and pH values were higher in the dry season upstream. Partially similar results were reported by da Silva et al., in the Rio Formoso estuary (Pernambuco, Brazil): They detected the highest pH values in the lower estuary region, in addition to higher DO and salinity [44]. According to da Silva et al., salinity and oxygenation levels are influenced by tidal cycles and photosynthesis and respiration rates [44]. The neutralization capacity existing in the aquatic ecosystem due to the buffering effect prevents great variations in the pH; therefore, the maximum values were obtained in areas with more significant saline influence. In the Guapi- Macacu River, the highest salinity is expected at the mouth of the Guanabara Bay as it is a coastal segment, and its intensity may vary within the rainy season, which favors its dilution. The higher rates of DO and pH reflect a rainless season, with greater water transparency, favoring photosynthesis (which removes CO2) and, consequently, raising the pH of the water due to the consumption of H+ ions.
When analyzed individually, temperature and pH were the environmental attributes that were not related to the different segments of the river, but the periods of the year. The highest temperature in the rainy season corresponds to the hottest season of the year in South America. However, there is collinearity of environmental attributes. When presented in the reduced model and correlated with species abundance, pH is displayed as a variable with high significance for the downstream area, probably due to its higher value in the dry season. The association between the other analyzed variables, such as the predominance of increased transparency in the intermediate region of the river, contributed to a better distinction between the river segments in the dry and rainy seasons, corroborating the environmental characteristics of the Guapi-Macacu river in the Guapimirim APA, and serving as predictors for related fish species in this habitat. According to Blaber et al., fish from tropical estuaries are subject to a series of interactions of physicochemical and biological factors that determine their patterns of occurrence, distribution, and movement [21]. For these authors, in the Rio Formoso estuary (Pernambuco, Brazil), temperature, salinity, pH, and dissolved oxygen were higher in the lower estuarine zone and during the dry season. However, inefficient levels of DO can be observed in the area downstream of the river, mainly in the rainy season, coinciding with the lowest richness and abundance registered. For Edokpayi et al., concentration levels of DO below 5.0 mg/L impair aquatic life [45]. Furthermore, during the rainy season, the currents in the Guanabara Bay carry organic materials to and concentrate contaminants during the dry season in the area downstream of the Guapi-Macacu River, in addition to the sediment resuspension, which explains the lower oxygenation during the rainy season in this area.
The fish community of the Guapi-Macacu river
The fish community of the Guapi-Macacu River, within the Guapimirim APA and its Buffer Zone, has freshwater species with the presence of marine species, many of which are euryhaline. Marine species were concentrated in the lower part of the river (downstream), at the mouth of the Guanabara Bay, with some species migrating inland, such as common snooks, grey mullets, jenny mojarras, and Whitemouth croakers. This segment consisted of resident species, marine and freshwater migrants, which use estuaries as feeding areas, for rearing larvae and juveniles, or for reproduction [46]. These habitats favor the presence of several fish populations on their shores, consisting mainly of juveniles of marine species [47,48]. Thus, the greater abundance of fish downstream of the Guapi-Macacu River is probably due to the availability of food from primary production, the structural complexity of the mangrove vegetation which provides refuge, especially for young fish and the high turbidity of the water, as well as for being a nursery for coastal species.
In estuarine environments, mangroves provide a natural refuge for young individuals due to the protection supplied by the root structure of their trees. Most fish caught in tropical coastal areas enjoy this protection during the juvenile phase and at the time of spawning and, therefore, are closely dependent on the integrity of this ecosystem [49]. Thus, the area with the highest occurrence of native species is also located downstream of the river, within the Guanabara ESEC, the most preserved area within the Guapimirim APA and with the best ecological indices, such as abundance and richness of the species found, mainly in the dry season. For Teixeira et al., the determination of biodiversity, especially of the fish community and its patterns of spatial and temporal variation, is of great relevance for the assessment of environmental quality [50].
Siluriformes was the most abundant fish order in the Guapi-Macacu River. The dominance of Siluriformes over others is a characteristic pattern of Brazil’s eastern region, being particularly accentuated in areas of high river courses, where the high hydrodynamic condition favors the occupation by demersal species [51]. Genidens genidens, a representative of the second most abundant family Ariidae occurs in coastal areas and is generally more significant in shallow coastal waters, on muddy or sandy bottoms [52-54]. The presence of G. genidens downstream from the river may be related to the spawning season. The species seek the mouth of rivers with the males and rarely females performing oral incubation and carrying eggs and initial forms of the offspring until they complete embryonic development; this explains the presence of specimens downstream of the Guapi-Macacu River. Furthermore, G. genidens exhibited high levels of fidelity (59.09%) and specificity (100%), considered a perfect indicator species in the IndVal analysis [55]. Thus, the IndVal analysis determined that for this specific area, regardless of the season analyzed, three species were considered indicators of this habitat: G. genidens, B. pectinate, and M. furnieri (Figure 8A). In addition to these species, E. saurus was selected only for the dry season in the downstream area. All species selected for the downstream area were considered asymmetric indicators, as they contribute more to habitat specificity than to fidelity [56,57]. This segment was also particularly evident in the cluster analysis, which showed that the main species selected in IndVal share this river segment.
The area within the Guapimirim APA, disregarding the Guanabara ESEC (i.e., the intermediate segment of the river), presented ecological indices similar to the other stretches of the river. However, there is greater abundance and richness (Figure 8A). Thus, the downstream and intermediate segments (i.e., comprising the Guanabara ESEC and Guapimirim APA) showed only C. undecimalis as an indicator of the APA regardless of the time of year assessed (Figures 8A and 8B). Centropomus undecimalis belongs to the order Perciformes, the second most abundant in the Guapi-Macacu river. According to Peterson et al., common snooks do not undergo major migratory cycles, being a relatively fast-growing fish that spawn large numbers of eggs in brackish waters during late spring and early summer [42]. Juvenile common snooks have a great affinity for fresh water and have higher survival rates than adults in waters with lower oxygen levels, being found upstream of rivers at all times of the year [58]. Its nursery or primary habitat has been described as shallow warm streams or drainage canals, with low current speeds and non-vegetated bottoms or at the border of mangroves [59]. As they develop, they move from habitats with shallow water to estuaries, mangroves, and deeper waters [60]. According to cluster analysis, the river segment shared with the C. undecimalis and other species provides this species with a habitat with abundant food resources and protection for its development.
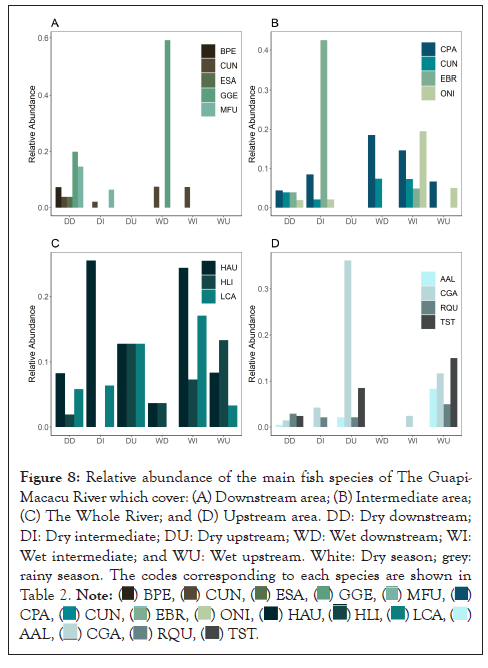
Figure 8: Relative abundance of the main fish species of The Guapi-Macacu River which cover: (A) Downstream area; (B) Intermediate area; (C) The Whole River; and (D) Upstream area. DD: Dry downstream; DI: Dry intermediate; DU: Dry upstream; WD: Wet downstream; WI: Wet intermediate; and WU: Wet upstream. White: Dry season; grey: rainy season. The codes corresponding to each species are shown in Table 2.
The intermediate region of the river was characterized by a very sinuous zone with greater diversity in physiography, constantly flooded, with deeper portions, and a salinity gradient that decreases from the river mouth to the interior. This segment has vegetation composed of mangrove forests, of the riverine type. This area is directly related to samples with greater transparency, showing E. brasilianus and O. niloticus with intermediate values of this attribute (Figure 8B). Eugerres brasilianus is a species of marine origin that tolerates significant variations in salinity [61]. It is anadromous, migrates from the sea to rivers, lives in coastal waters of warm seas, and penetrates coastal lagoons and estuaries to complete its life cycle [50]. Furthermore, E. brasilianus is a nocturnal, generalist, and opportunistic species; it is also epibenthic and demersal. That is, it presents patterns strictly linked to the substrate, being considered an excellent biological resource, mainly because it is regarded as an abundant fishery resource.
On the other hand, the cluster analysis did not show the intermediate region with a specific community for this segment of the river, presenting itself as a transition area, occupied mainly by species that travel along the river, such as H. littorale, Hypostomus auroguttatus and Loricariichthys castaneus (Figure 8C). Hypostomus auroguttatus and L. castaneus belong to the most abundant family the Loricariidae common in areas with muddy river bottoms and may occur even in lentic environments. These two species recorded in the three segments of the Guapi-Macacu River during the dry and rainy seasons were considered generalists in this study.
The species A. lacustris of Figure 5, considered rare in the analysis presented in the CLAM TEST, was selected by IndVal for the stretch upstream of the river, as well as in the rainy season, with high specificity and low fidelity. For Dufrêne et al., rare species can get the same IndVal value as indicator species and are called asymmetric indicators [29]. On the other hand, in the same river stretch, disregarding the attributes of the seasons (dry or rainy), IndVal selected T. striatulus and C. gariepinus in Figure 8D, both with high specificity, which means that these fish can also be considered asymmetric indicators, contributing to habitat specificity, but are not useful to predict groups [29].
From the CCA, it is noticed that the stretch upstream of the river presents higher values of DO and an abundance of C. gariepinus. The upstream part is the headwaters of the river, which have a humid tropical climate, and high and variable slope, determining the dynamic character of the fluvial system, with the presence of rapids, characteristic of mountainous and plateau regions. Riparian forests are a key factor in providing resources for feeding aquatic fauna and attracting dispersers, making the riparian environment a fundamental element in the sustainability of rivers and lakes and in the connection between the different systems that compose them. The CCA also highlights the most protected area of the Guapimirim APA, downstream of the river, with greater diversity and richness, presenting greater attributes of salinity, pH, and turbidity. In general, the most abundant species in river flow are intermediate values of the abiotic variables analyzed, except for C. gariepinus, which correlates with higher dissolved oxygen samples, and M. furnieri, which presents more significant correlations with salinity values. C. parallelus, H. auroguttatus, H. littorale, and L. castaneus express abundances related to intermediate values of transparency, showing correlations in all river segments. As well as the CCA, the cluster analysis also evidenced the group formed by G. genidens, B. pectinata and M. furnieri, revealing proximity of sharing in the same region, mainly in the dry season, with a dominance of G. genidens, as it presents greater abundance in this group. The CCA also highlights the similar spatial distribution of T. striatulus with C. gariepinus (with greater representation in abundance), corroborating the cluster analysis.
African sharptooth catfish in the Guapi-Macacu river
The African sharp tooth catfish (C. gariepinus) is among the most abundant species in the river, but it still does not show a significant abundance in the most preserved area of the Guapimirim APA. The species that present ecological equivalence to the African sharp tooth catfish, namely the R. quelen and the T. striatulus, despite having a similar distribution, presented lower abundance, which denotes the overlapping of IAS’ habitat over the native’s habitat (Figure 9A). In addition, the population of C. gariepinus presented higher contributions of abundance in the dry season, mainly upstream, compared to the other species, demonstrating a high potential to colonize the entire river (Figure 9B).
Although the CCA analysis does not show similar correlations of the abiotic factors R. quelen with Clarias gariepinus, the group exposed in the cluster analysis, it is clear that these species share the same river segments. Rhamdia quelen prefers lakes and river bottoms, selecting environments with calmer water and sandy, muddy bottoms, along the riverbanks and vegetation. They are omnivore’s species with a clear preference for fish, crustaceans, insects, plant remains, and organic debris; therefore, they are considered generalists in terms of food choice [31]. The R. quelen and the C. gariepinus share many biological similarities, especially the food preference and habitat use; however, the R. quelen has a disadvantage in terms of its development, as females can reach up to 66.5 cm and males up to 52.0 cm [31].
According to our analysis, dissolved oxygen was the attribute directly correlated with the distribution of C. gariepinus (Figure 7B). Thus, Dissolved Oxygen (DO) plays a key role in regulating the metabolic functions of the organism, including the aquatic community, in addition to being an environmental indicator of water quality. On the other hand, the African sharp tooth catfish can tolerate low concentrations of dissolved oxygen due to an accessory air-breathing organ that can absorb oxygen from atmospheric air, allowing it to survive out of water for long hours or even weeks or even in muddy swamps.
Another significant environmental attribute in our analysis was the temperature, which seems to be an essential factor in the distribution of the species in the Guapi-Macacu River. The highest temperature values in the upstream area corroborate the results found in the CCA, where the highest abundance of the species was correlated with this stretch of the river. For Hecht et al., the African sharp tooth catfish larvae have an optimal development around 28˚C, a value that was recorded in the rainy season upstream [32]. Indeed, it could be observed during the dry season, when there was a large increase in the population of the IAS, favored by the increase in temperature recorded previously. On the other hand, our study indicates that, although the average temperature values measured in the Guapi-Macacu River are not considered ideal for the development of the catfish, the species’ adaptability allowed it to develop well in this ecosystem and colonize other segments of the river. However, climate change resulting from global warming may contribute to a gradual increase in temperature, significantly altering this scenario, which may favor the rapid development of this alien species and, possibly, the decline of native populations.
Salinity is also an attribute that limits the occurrence of the alien species at the mouth of the Guanabara Bay. De Melo et al., detected an intrusion of salinity into the river in the dry season of the year, corroborating our findings, where salinity varied considerably in this season, reaching 31 [28]. This factor may have contributed to the lower abundance of the IAS in this stretch of the river. This occurs because African sharp tooth catfish are stenohaline, with limited ability to tolerate increased salinity in the environment at later stages. Borode et al., conducted a study on the effect of salinity on the early development of the African catfish [23]. They concluded that increased salinity delays the hatching and development of African sharp tooth catfish eggs and larvae, but the African catfish can tolerate variations of up to 6 ppt for its growth. Even so, despite the significant abundance of this species, the specificity and fidelity analysis does not list the C. gariepinus as an indicator species, considering both the seasons studied and the collecting areas. However, this pattern diverges when considering only the upstream zone, when the species specificity index rises to 81% and fidelity to 36% for this area, demonstrating that the C. gariepinus has a probability of 53% to be an asymmetric indicator species of this area. For Dufrêne et al., a species can be an asymmetric indicator without high fidelity [29].
The analyses presented herein contributed to the knowledge of the ichthyofauna of the Guapi-Macacu River and how they may help future studies of the impacts related to the presence of the alien species. Even so, our study reveals that this species still does not colonize the Guanabara Bay, or the downstream portion of the river. These factors may be related to a lower temperature, higher salinity, and potential predators and competitors that occupy this stretch of the river. In addition, we show that this conservation area still fulfills its role of protecting the resident ichthyofauna, harboring a larger contingent of typically estuarine juvenile fish, which portrays this area as a natural breeding ground and shelter for several species of fish of ecological and economic importance. However, the gradual increase in temperature in aquatic ecosystems is one of the factors that may favor the development of African sharp tooth catfish. Southeast Brazil has been experiencing more intense summers and prolonged periods of droughts, and consequently, an increase in the temperature in stretches of the river that may contribute to the successful development of African sharp tooth catfish. Moreover, the IAS has been competing for habitats with native species that have ecological equivalence, such as R. quelen, and T. striatulus, thus impacting the local ichthyofauna, with a visible decline in the abundance of native species where the African catfish settles. Therefore, it is necessary to constantly monitor key species, such as G. genidens and C. undecimalis, as well as the population of the invasive species in this area of environmental preservation.
This study was performed within the scope of the project entitled “The Invasion of African Sharptooth Catfish in the Guanabara Bay–RJ”, contemplated by the National Council for Scientific and Technological Development (CNPq), Brazil, Universal Public Notice no. 405984/2016-2. The author Michelle Torres Dumith received support from the Coordination for the Improvement of Higher Education Personnel (CAPES).
Citation: Dumith MT, Santos AFGN (2022) Ichthyofauna Structure at Risk due to the Bio Invasion of Clarias gariepinus in a River at Guapimirim Environmental Protection Area, Southeastern Brazil. Fish Aqua J. 13: 305.
Received: 16-Aug-2022, Manuscript No. FAJ-22-18867; Editor assigned: 19-Aug-2022, Pre QC No. FAJ-22-18867 (PQ); Reviewed: 02-Sep-2022, QC No. FAJ-22-18867; Revised: 09-Sep-2022, Manuscript No. FAJ-22-18867 (R); Published: 16-Sep-2022 , DOI: 10.35248/2150-3508.22.13.305
Copyright: © 2022 Dumith MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.