Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2022)Volume 13, Issue 6
Aspirin (Acetylsalicylic acid; ASA) an anti-inflammatory and anti-cancer molecule derived from Salicylic Acid (SA) use to relieves pain, swelling, cold or flu, prevent colorectal cancer and cardiovascular diseases. ASA induces defense response against wide range of pathogens in plants. Recently, we reported Benzoyl Salicylic Acid (BzSA) and other anti-cancer molecules such as Gallic Acid (GA) methyl gallate from the seed coats of G. rottleriformis. The present study reporting the detection of natural aspirin for the first time in the seed coats, leaves and bark of G. rottleriformis. HPLC Separation chromatogram of hexane extract of seed coats, leaves and bark shown a peak at RT 24.8 min coeluted with the aspirin standard. The purified aspirin from the seed coats were subjected to 1H NMR and were confirmed as aspirin. These results suggest that aspirin biosynthesis is taking place in seed coats, leaves and bark of G. rottleriformis and supports the medicinal properties of G. rottleriformis. These results suggest that the seed coats, leaves and bark of this plant contains the highly useful medicinal compounds to treat the rheumatism, cancer, cardiovascular, psoriasis, anti-inflammatory and other skin diseases.
Benzoylsalicylic acid; Acetylsalicylic acid; Salicylic acid; Cyclooxygenase; IκB kinase; plant defense
BzSA: Benzoylsalicylic Acid; SA: Salicylic Acid; ASA: Acetylsalicylic Acid; COX: Cyclo-Oxygenase; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; PG: Prostaglandin
Givotia rottleriformis is a commercially valuable tree belonging to the Euphorbiaceous family. It is a moderate sized tree grows in the forests of Andhra Pradesh, Karnataka, Tamil Nadu, and West Bengal [1]. Because of the softness and light weight of the wood this plant has high value in toy industry. And the seeds and the bark have medical value and used for the treatment of rheumatism, dandruff, and psoriasis [2]. Aspirin also known as Acetyl Salicylic Acid (ASA) and it is a nonsteroidal anti-inflammatory drug and it is a commonly used drug worldwide to reduce pain, fever and inflammation. Aspirin is a potential drug used to prevent cardiovascular and cerebrovascular diseases [3]. About 80 million pounds of aspirin are producing and 100 billion tablets consumed every year. Aspirin primarily works by blocking the action of cyclooxygenase1, which prevents the conversion of Arachidonic Acid (AA) into Prostaglandins (PG), which further prevents the synthesis of Thromboxane (Tx) [4-6]. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) blocks the COX enzymes and reduce prostaglandins throughout the body and thus reduce inflammation, pain, and fever [7,8]. PG is produced by the cells by the enzyme Cyclooxygenase (COX) and carry out several important functions. After more than a century of human use, researchers are still discovering how aspirin affects the body and the Scientists understood that SA was the component derived from plants that relieved pain fever and cancers [9-13]. However, long-term use of ASA in high doses causes stomach problems in some people [14]. In 1897 a chemist at a company called Bayer added a chemical modification called an acetyl group (CH3CO) to SA, turning it into ASA and Bayer called this new substance aspirin [15]. Willow bark has been used as a traditional medicine for more than 3500 years and determined salicylate contents in the food items [12,15-18]. For centuries in Europe, people grew meadowsweet to treat pain and inflammation [15,16,19,20]. Willow and meadowsweet contain high levels of aspirin-like compounds called salicin and Methyl Salicylate (MeSA) which would later form the basis for the discovery of aspirin [15,16]. The effect of aspirin still studying with the growing evidence of its chemo preventive effect against colorectal and other types of cancer [15,21-25].
Salicylic acid pathway is a well-studied defense responsive pathway against a broad range of bacterial, fungal and viral pathogens [26-28]. It is widely accepted that plants possess both an Isochorismate Synthase (ICS) and Phenylalanine Ammonia-Lyase (PAL) pathway to synthesize SA both starting from chorismite [27,29]. SA levels increase in many plants upon infection with viruses, fungi, insects, and bacteria and exogenous SA ptr-treatment boosts the defense system of the host plants [30-34]. Plants overexpressing NahG, a salicylate hydroxylase converts SA to catechol, are unable to accumulate SA upon pathogen infection and are impaired in their Systemic Acquired Resistance (SAR), a broad-spectrum systemic defense response after a primary infection [35].
One of the most important factors that dictates the distribution of many plant species is their ability to withstand environmental stress including seasonal variations in temperature and available moisture [36,37].The hypothesis that physiologically active concentrations of Salicylic Acid (SA) and its derivatives can confer stress tolerance in plants [38]. Plants grown from seeds imbibed in aqueous solutions (0.1–0.5 mm) of SA/ASA displayed enhanced tolerance to heat, chilling and drought stresses [39,40]. Seedlings acquired similar stress tolerance when SA or ASA treatments were applied as soil drenches [41]. The fact that seed imbibition with SA or ASA confers stress tolerance in plants and it is more consistent with a defense signaling role of SA and ASA [42,43]. Induction of multiple stress tolerance in plants by SA and its derivatives are useful in agriculture, horticulture and forestry [39,43]. SA/ASA provides multiple stress tolerance by regulating the expression of stress responsive genes in plants [43]. SA or ASA imbibed into a seed impart tolerance to a variety of stresses in plants [44,45]. In our previous study we have shown that pretreatment of SA, ASA, and BzSA (Benzoylsalicylic Acid) to tobacco induced SAR and offered better protection against tobacco mosaic virus [46,47].
In the present study we have identified and purified natural aspirin from the seed coats, leaves and bark of G. rottleriformis using HPLC using preparative column (Figure 1). The purified aspirin coeluted with the standard aspirin at RT 24.8 min and the purified aspirin was confirmed by 1H NMR. Pre-treatment of tobacco plants with purified natural aspirin shown the similar effect in reducing TMV lesions as compared to standard ASA.
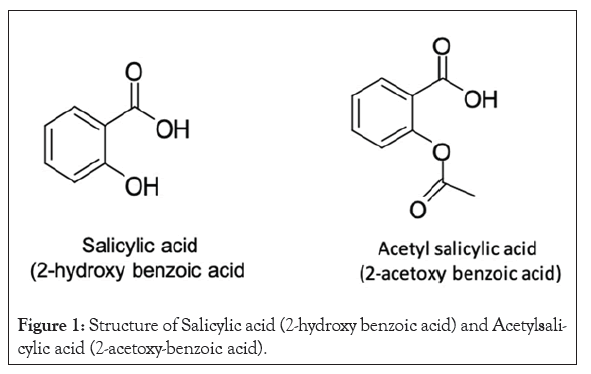
Figure 1: Structure of Salicylic acid (2-hydroxy benzoic acid) and Acetylsalicylic acid (2-acetoxy-benzoic acid).
Plant material
Mature, dry seeds, bark and leaves of G. rottleriformis (A Voucher Specimen No. PARC/2011/2140) were collected from Regional Forest Research Centre (RFRC), Rajahmundry, Andhra Pradesh, India. Tobacco seeds (VT-1158, NN gene type, resistant to TMV) were obtained from the Central Tobacco Research Institute, Rajahmundry, Andhra Pradesh, India.
Compound extraction from seed coats, leaves and bark
Mature seeds leave and barks of G. rottleriformis were dried at room temperature for 3 days, the seed coat consisting of epicarp and mesocarp were removed manually using cutter. Dried seed coats, leaves and bark were ground into a fine powder separately using a grinder. The powder was soaked in methanol repeatedly for 3 times for 2 days and the total compounds were extracted. The extract was vacuum dried with the help of rotary evaporator with vacuum under the heating condition.
Open silica column chromatography
Slurry of seed coats, leaves and bark was prepared separately from semi solid extracts by adding silica and applied to open silica column. The separation of compounds was carried out by changing the polarity low to high of the mobile phase as hexane and ethyl acetate. Each fraction eluted from the open silica column and subjected to tin layer chromatography to check the purity of the fractions. A fraction number 3 eluted from open silica column with 10% ethyl acetate (10 ml ethyl acetate and 90 ml hexane) was concentrated and subjected to reverse phase HPLC using C18 silica column.
HPLC purification of aspirin from the seed coats of G. rottleriformis
The concentrated open silica column fractions were dissolved in HPLC grade methanol and subjected to reverse phase HPLC employing C18 silica column (Shim pack Column 250 × 4.6 mm and particle size 5 µm) with flow rate of 6 ml/min, UV detection at 280 nm and mobile phase of solvent A (water: acetic acid 1000:1 and solvent-B methanol : acetic acid 1000:1 and applied in a gradient program (0-5 min, 55% B linear; 5-20 min, 95% B linear; 20-25 min, 95.5% A, 25-30 min, 5.5% A stop). The eluted peaks were collected and concentrated by lyophilisation.
HNMR analysis of purified Aspirin
HNMR (400 MHz) spectra were recorded on Bruker-Avance-400 spectrometer with chloroform-d as solvent and TMS as reference (d=0 ppm). The chemical shifts were expressed delta in downfield from the signal of internal TMS.
We have initiated out study to re-establish G. rottleriformis plant populations, and developed an efficient micro propagation method [1]. While cutting the seeds we observed a bulk amount of compound in the seed coats. Based on the medicinal properties of this plant, we have directed to characterize the medicinally important compounds form the seed coats, leaves and the bark of this plant. And we isolated important bioactive molecules [46-48]. In our previous studies, we repot Benzoyl Salicylic Acid (BzSA) for the first time from the seed coats of G. rottleriformis and proved as a potential defense inducer against Tobacco Mosaic Virus (TMV) as a compared to salicylic acid a well-known plant hormone induces disease response against a wide range of pathogens [46]. BzSA, Salicylic Acid (SA) and its precursors such as Cinnamic Acid (CA), Benz aldehyde (BD) and Benzoic Acid (BA) are purified from the seed coats of G. rottleriformis using preparative HPLC [46]. The biosynthesis of SA was reported in different plants are takes place via cinnamic acid [29,49]. In our previous study we report that SA is further converted to BzSA using benzoyl-CoA [46]. In addition we also reported the purified Gallic Acid (GA) and Methyl Gallate (MG) from the seed coats of G. rottleriformis [48]. The presence of GA, MG, SA, SA-analogues and BzSA in the seed coats are suggested that the existence of phenylpropanoid pathway in the seed coats of this plant [46-48]. A peak eluted at RT 24.8 min from the 10% fraction of seed coats co-eluted with standard aspirin (Figure 2). In order to determine the presence of aspirin in the leaves and bark of this plant, A 10% fraction of leaf/bark from the open silica column were resolved on preparative HPLC using the same HPLC program and the HPLC chromatogram showed the elution of peak at RT 24.8 min corelates with the standard aspirin (Figures 3 and 4). The peak eluted at RT 24.8 min was purified and subjected to 1H NMR and confirmed as aspirin (Figure 5). Tobacco plants that were pre-treated with purified natural ASA showed similar effect in decrease in lesion size as compared with standard ASA (Figure S1). The amount of aspirin detection was significantly high in the leaves as compared with the seed coats and the bark (Figure 2 and 4). SA and related compounds are produced by plants as part of their defense systems against pathogen attack and environmental stress [16]. So far, no reports on the biosynthesis of aspirin in the plants are published. First time we are reporting the aspirin in the seed coats, leaves and the bark of G. rottleriformis. HPLC analysis of fruits and vegetables provide unknown amounts of aspirin and no aspirin detected in foods by HPLC [17]. It was reported that ASA concentrations were too low in volunteers eating a verity of diets [11,17,50,51]. Our results strongly suggest that the biosynthesis of aspirin is taking place in the seed coats, leaves, and the bark of this plant. The biosynthesis of aspirin in plants requires SA as a precursor was detected in the seed coats [46]. BzSA purified from the seed coats of this plant also required SA as a key precursor [46,47]. The chemical synthesis of BzSA was achieved using SA and benzoyl chloride [47]. Previous reports suggest that pre-treatment of aspirin induces Systemic Acquired Resistance (SAR) in plants against a broad spectrum of pathogens [46,47,52-54]. Aspirin has been used for >100 years for pain relief and to treat inflammatory conditions and fevers [55,56]. Aspirin is effective in the prevention of cardiovascular disease and several cancers [57-64]. In the 1990's an important discovery was made from elegant molecular and cellular biological studies that there are two Cyclo-Oxygenase (COX) enzyme systems controlling the production of prostanoids {Prostaglandins (PGs) and Thromboxane (TxA2)}; COX-1 that produces PGs and Thromboxane A2 that regulate gastrointestinal, renal, vascular and other physiological functions, and COX-2 that regulates production of PGs involved in inflammation, pain and fever [55,65-68]. The stage was set in the 1990's for the discovery and development of drugs to selectively control COX-2 and spare the COX-1 that is central to physiological processes whose inhibition was considered a major factor in development of adverse reactions, including those in the gastro-intestinal tract [69]. It has been evaluated in over 150 randomized controlled trials and a small daily dose of around 100 mg has been shown to reduce the risk of myocardial infarction and stroke by about 30% [70]. The constitutive isoform, COX-1, supports the beneficial homeostatic functions, whereas the inducible isoform COX-2 becomes unregulated by inflammatory mediators and its products cause many of the symptoms of inflammatory diseases such as rheumatoid and osteoarthritis [68,71,72]. BzSA purified from the seed coats of this plant inhibits higher COX-2 than COX-1(data not shown) and the existence of aspirin in these plants supporting the anti-rheumatism medicinal properties of this plant [68,73]. The seeds and bark of this plant used for the treatment of psoriasis [74-76]. A Randomized trial of low-dose Aspirin to reduce vascular endothelial Inflammation in Psoriasis [74-76]. The plant G. rottleriformis grown particularly in hill forests and exposed to high temperatures may be because of high temperature stress and other abiotic and biotic stresses this plant synthesizes a lot of stress and other compounds to overcome biotic/abiotic stresses. Several studies reported that SA and ASA protect plants from biotic/abiotic stresses both in plants and animals [36,77-80]. A 13C6-benzoic acid load ingested by six volunteers led, between 8 and 16 h, to a median 33.9% labelling of urinary salicylic acid and SA appears to be, at least partially, an endogenous compound should lead to reassessment of its role in human and animal pathophysiology [81]. Aspirin is very rapidly hydrolysed, having a plasma t1/2 of 20 min, to the deacetylated metabolite SA (2-hydroxybenzoic acid), which has a half-life of between 2 and 4 h. Aspirin absorption follows first-order kinetics with an absorption half-life ranging from 5 to 16 minutes [82]. Hydrolysis of aspirin to SA by nonspecific esterase’s occurs in the liver and, to a lesser extent, the stomach so that only 68% of the dose reaches the systemic circulation as aspirin [82]. Both aspirin and SA are bound to serum albumin (aspirin being capable of irreversibly acetylating many proteins), and both are distributed in the synovial cavity, central nervous system, and saliva [82]. The serum half-life of aspirin is approximately 20 minutes [82]. Acetylation of COX-1 and COX-2 IKK-complex inhibit the enzyme activity and are important to treat pain, fever, inflammation, cardiovascular and various cancers and the excess of aspirin deacetylate into salicylic acid and excreted [68,83]. The fall in aspirin concentration is associated with a rapid rise in SA concentration [84]. SA is renally excreted in part unchanged and the rate of elimination is influenced by urinary pH, the presence of organic acids, and the urinary flow rate [11,85].
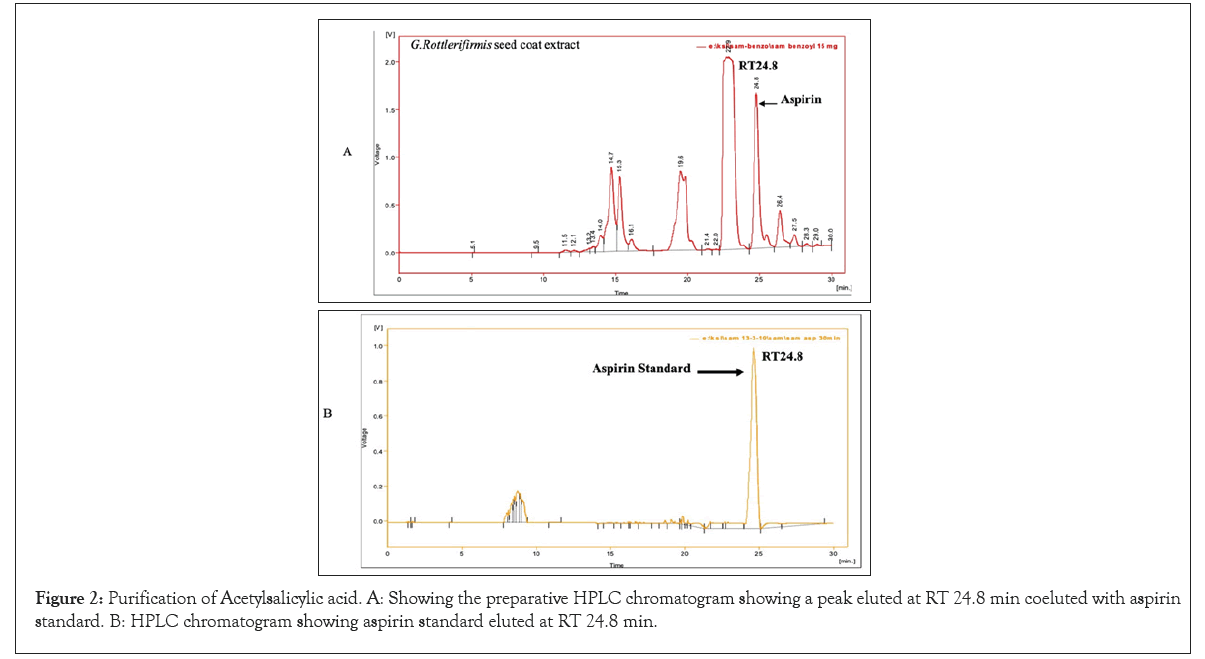
Figure 2: Purification of Acetylsalicylic acid. A: Showing the preparative HPLC chromatogram showing a peak eluted at RT 24.8 min coeluted with aspirin standard. B: HPLC chromatogram showing aspirin standard eluted at RT 24.8 min.
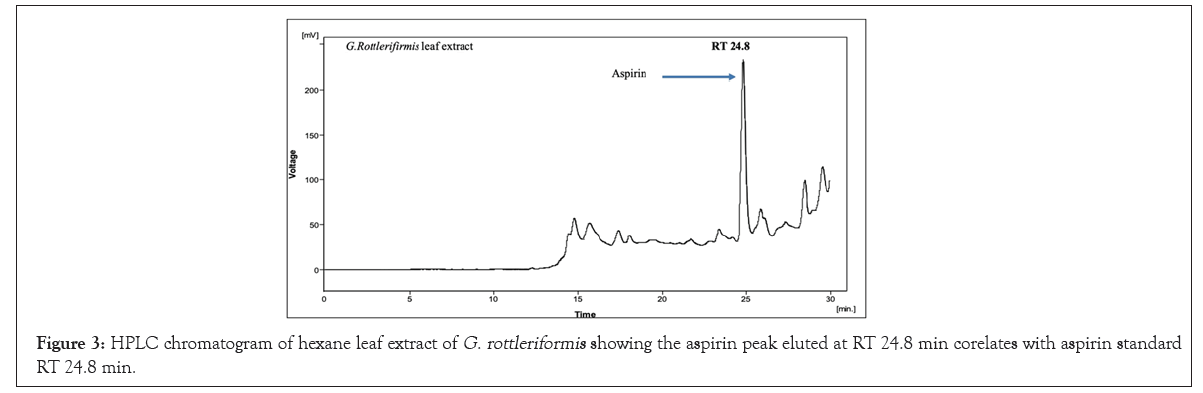
Figure 3: HPLC chromatogram of hexane leaf extract of G. rottleriformis showing the aspirin peak eluted at RT 24.8 min corelates with aspirin standard RT 24.8 min.
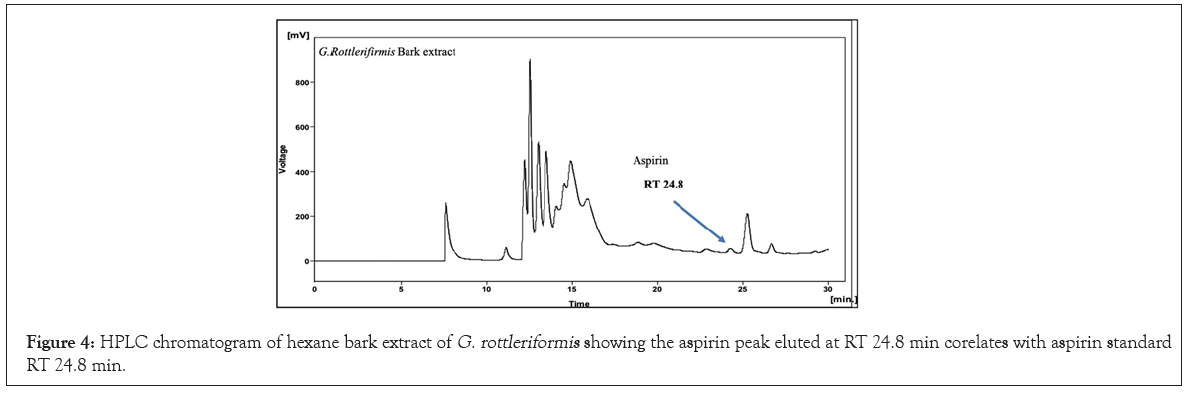
Figure 4: HPLC chromatogram of hexane bark extract of G. rottleriformis showing the aspirin peak eluted at RT 24.8 min corelates with aspirin standard RT 24.8 min.
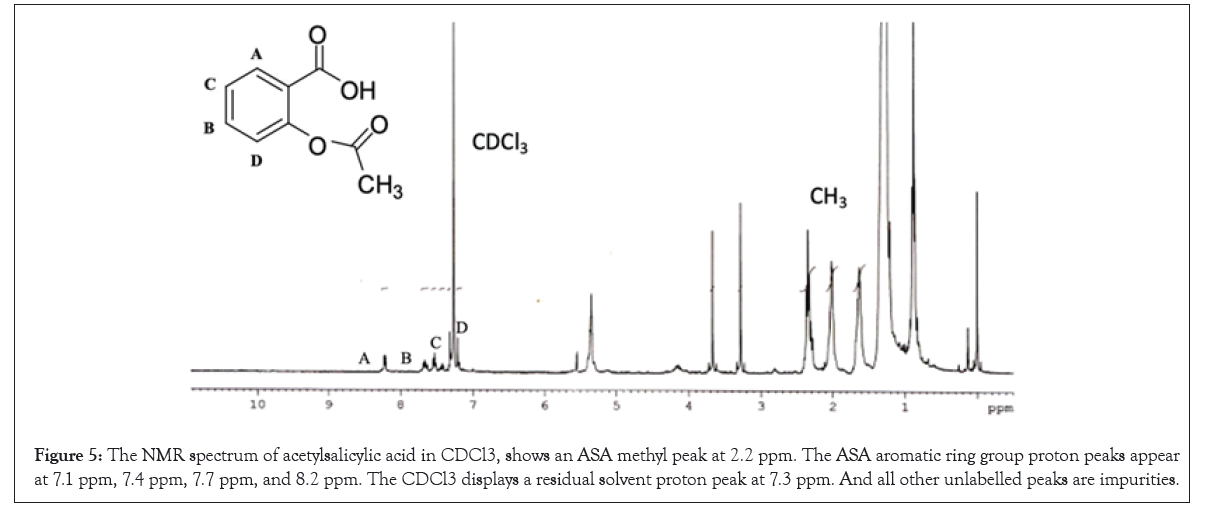
Figure 5: The NMR spectrum of acetylsalicylic acid in CDCl3, shows an ASA methyl peak at 2.2 ppm. The ASA aromatic ring group proton peaks appear at 7.1 ppm, 7.4 ppm, 7.7 ppm, and 8.2 ppm. The CDCl3 displays a residual solvent proton peak at 7.3 ppm. And all other unlabelled peaks are impurities.
Our results summarizing that identification of natural aspirin in G. rottleriformis suggest that both plants and animals utilizing aspirin and use of salicylates in rheumatic diseases support the medicinal properties of G. rottleriformis. And identification of SA, ASA, BzSA, BA, BD, GA and MG in this plants indicating that phenylpropanoid biosynthesis pathway is highly active in this plant and based on our results we suggesting that seed coats, leaves and the bark of G. rottleriformis contains aspirin and are useful to treat cancer, cardiovascular anti-inflammatory, psoriasis and rheumatic diseases.
Dr. Samuel Kamatham gratefully acknowledges the Science and Engineering Research Board (SERB), India for the grant received under Fast Track Young Scientists Scheme. I express our thanks to Dr. Vara Prasad, Regional Forest Research Centre, Rajahmundry for permitting us to collect the plant material for the study. KS gratefully acknowledges Prof. Pallu Reddanna, Department of Animal Sciences, School of Life Sciences, University of Hyderabad for allowing me to use needful lab facilities. Finally, we acknowledge the facilities at the School of Life Sciences, University of Hyderabad established with the support of UGC-SAP-CAS, DST-FIST and DBT-CREBB.
There is no conflict of interest.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Kamatham S (2022) Identification and HPLC Purification of Aspirin (Acetylsalicylic acid) from the Seed Coats, Leaves and Bark of Givotia Rottleriformis, J Chromatogr Sep. 13:492
Received: 19-Aug-2022, Manuscript No. JCGST-22-18914; Editor assigned: 22-Aug-2022, Pre QC No. JCGST-22-18914 (PQ); Reviewed: 06-Sep-2022, QC No. JCGST-22-18914; Revised: 13-Sep-2022, Manuscript No. JCGST-22-18914 (R); Published: 20-Sep-2022 , DOI: 10.35248/2157-7064.22.13.492
Copyright: © 2022 Kamatham S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.