Research Article - (2016) Volume 2, Issue 4
Identification of Endophytic Bacteria and their Characterization as Biocontrol Agents against Tomato Southern Blight Disease
*Corresponding Author: Claudia Mónica Ribaudo, Facultad de Agronomía, Universidad de Buenos Aires, Cátedra De Bioquímica, Avenida San Martín 4453, C1417DSE Buenos Aires, Argentina, Tel: +54 11 4524 8087 Email:
Abstract
Objectives: The purpose of the present study was to characterize the endophytic bacterium PAC BNM0522 and to evaluate its role in the ethylene signaling pathway in the development of southern blight disease (caused by the necrotrophic fungus Sclerotium rolfsii) in tomato plants. Methods: In this study, biochemical tests, 16S rRNA gene sequencing, and amplification of the ACCD gene (acdS) were performed on the isolate obtained from pruning compost. This isolate was also screened for antibiotic production and competition for nutrients and evaluated its role in the ethylene signaling pathway by analysis of the expression of genes involved in ethylene biosynthesis by PCR and ethylene content by GC. Results: The analysis of the 16S rRNA gene partial sequence and biochemical test placed this bacterium within the genus Pseudomonas and revealed that it belongs to the group of PGPR with multiple plant growth-promoting traits. This rhizobacterium was able to promote host growth through direct antagonism to the fungal pathogen. The in vivo experiments showed that PAC BNM0522 inoculation controlled the disease caused by S. rolfsii. The ethylene level was enhanced in tomato plants infected with the fungus and decreased by inoculation of the rhizobacterium. The expression of the Sl-ACS2 isoform was induced by the presence of the pathogen and strongly repressed in PAC BNM0522-primed plants. The accumulation of the Sl-ACO1 messenger was down-regulated in deaminaseproducing PAC BNM0522-inoculated plants. Conclusion: Our results indicate that ACCD-containing PAC BNM0522 generated protection of tomato plants under pathogenic stress and alleviated ethylene-induced damage, by regulating the expression of genes involved in the ethylene pathway. PAC BNM0522 may be used as an alternative ecological approach to control soil-borne fungi.
Keywords: ACC deaminase; Biocontrol; Ethylene; Plant growthpromoting rhizobacteria; Solanum lycopersicum
Introduction
To cope with the hostile environment where a plethora of pathogenic microorganisms such as fungi, bacteria, and viruses can cause disease, plants have evolved a sophisticated defense system. It is generally accepted that hormones such as salicylate, oxylipin, jasmonates and ethylene play major roles in regulating induced defense responses [1,2]. Following pathogen infection, many of the symptoms observed in an infected plant arise as a direct consequence of the increased production of stress ethylene induced by the pathogen [3,4]. In fact, the use of chemicals to either increase or inhibit the synthesis or perception of ethylene and the use of mutants altered in the ethylene signaling pathway have demonstrated that ethylene can improve the ability of several pathogens to colonize plant tissues [5]. In the ethylene biosynthetic pathway, the enzymes 1- aminocyclopropane-1-carboxylate synthase (ACS, EC 4.4.1.14) and 1- aminocyclopropane-1-carboxylate oxidase (ACO, EC 1.14.17.4) catalyze the reaction from S-adenosyl methionine to 1- aminocyclopropane-1-carboxylate acid (ACC) and from ACC to ethylene, respectively, and the biosynthesis is subject to both positive and negative feedback regulation [6]. So, any biological or chemical treatment that can lower the magnitude of stress ethylene should decrease the damage to the plant. Certain non-pathogenic rhizobacteria, known as plant growth-promoting rhizobacteria (PGPR) have shown ability to promote plant growth and increase productivity by various mechanisms [7-9]. Many PGPR contain the enzyme ACC deaminase (ACCD) (EC 4.1.99.4), which cleaves ACC to form α- ketobutyrate and ammonium, thereby lowering the levels of ethylene, and may thus be responsible for alleviating the stress caused by increased levels of ethylene. The pretreatment of plants with ACCDcontaining PGPR can provide significant protection to plants against the damage caused by ethylene [7,9]. ACCD was first purified from the Pseudomonas sp. strain ACP [10] and retrieved from P. chlororaphis [11] and P. pseudoalcaligenes [12]. Lund et al. [13] demonstrated that a tomato transgenic line expressing ACCD from the Pseudomonas sp. strain 6G5 was less susceptible to infection with Xanthomonas campestris pv. vesicatoria, P. syringae pv. tomato and Fusarium oxysporum f.sp. lycopersici. These authors also demonstrated that both perception and synthesis of ethylene are necessary for disease development.
Among the known soil-borne fungi in plants, Sclerotium rolfsii is one of the main problems in tomato (Solanum lycopersicum ) cultivation in the warm regions of the world. Southern blight disease, caused by S. rolfsii , causes damping-off in tomato seedlings as well as basal stem rot, wilting and blight in adult tomato plants. S. rolfsii develops resistance structures (sclerotia) that allow long survival in the soil. Sclerotia germinate at the base of plants, giving a visible mycelial mat whose hyphae penetrate the plant tissues. S. rolfsii produces a battery of extracellular enzymes aimed to attack vegetable tissues and important amounts of oxalic acid that alter the pH of tissues, favoring the activity of fungal endopolygalacturonases and cellulases [14]. The use of fungicides to control these diseases is difficult due to the economic costs of implementation and the environmental problems that arise from the widespread use of toxic chemicals. As a result, new research has focused on developing safe and reliable means to improve crop protection against plant infection such as the use of parasitism by other fungi [15] or the use of PGPR [16].
The purpose of the present study was to characterize the endophytic bacterium PAC BNM0522 and to evaluate its role in the ethylene signaling pathway in the development of southern blight disease (caused by the necrotrophic fungus Sclerotium rolfsii) in tomato plants.
We hypothesized that ethylene synthesis is impeded in inoculated plants, leading to a delayed appearance of symptoms through a mechanism that involves transcriptional changes in genes of the ethylene pathway in response to bacterial ACCD activity.
Materials and Methods
Bacterial strains
The strain PAC BNM0522 was isolated from pruning compost of Platanus hispanica Mill. As described in Nico et al. [17]. Morphology and motility were determined and biochemical tests were performed.
Azospirillum brasilense FT326 (BR 11015) was provided by Dr Döbereiner and used as a control strain [17].
Bacterial identification
PAC BNM0522 was grown at 25ºC for 24 h in BHA (Merk, Germany). After lysing cells, the DNA was extracted using a microbial DNA isolation kit (Ultra Clean, Mo. Bio Laboratories, Carlsbad, CA, USA) as described in Nico et al. [17].
Cells were Gram-stained and examined for catalase and oxidase activity. Oxidase activity was measured using the reactive discs from Oxoid. Tests for gelatin liquefaction, starch hydrolysis and utilization of different sugars in O/F medium were conducted according to Levine and Carpenter [18] and Merck’s Microbiology Manual respectively. Biochemical tests were also performed and analyzed with the API 20 NE by RapIDTM NF Plus System (Remmel). The results were obtained after 4 h of incubation as recommended by the manufacturer. The pH range (1.5-13 at 30ºC) and salinity range (0-5% w/v, at 30ºC) for growth were recorded after incubation for 72 h on tryptic soy agar (TSA) supplemented with NaCl. The sensitivity of PAC BNM0522 to seven antibiotics was tested in Müeller Hinton agar by using antimicrobial susceptibility discs (Oxoid).
ACC metabolism assay
The ACC metabolism assay (qualitative) was carried out to characterize PAC BNM0522 for its ability to use ACC as the sole nitrogen source. The bacterium was grown on two nitrogen sources, ACC and (NH4)2SO4, to observe its growth rate in parallel with (NH4)2SO4. A. brasilense was used as control.
Bacteria were inoculated in tryptic soy broth (TSB) and incubated for 48 h at 30ºC. After that, 1 ml of each culture was collected and centrifuged at 5000 rpm. The pellet was resuspended in salt minimum medium DF salts supplemented with malate (5 mM) as carbon source [19] and adjusted until reaching the same OD550 nm. One set of tubes received (NH4)2SO4 (final concentration 10 mM), whereas another set received ACC (Sigma) (final concentration 0.5 mM) as nitrogen source. The uninoculated control tube received the same volume of sterile distilled water. The bacteria were incubated for 96 h at 30°C and OD550 was measured.
Amplification of the ACCD gene (acdS)
Cell extracts were prepared from cells grown in 20 ml TSB containing 3 mM ACC and incubated at 30ºC for 36 h. The bacteria were centrifuged at 5000 rpm and RNA was extracted using an RNeasy Protect Bacteria Mini Kit (Quiagen). First-strand cDNA was prepared using SuperScript II RNAse H-Reverse Transcriptase (Invitrogen) following the manufacturer´s specifications.
The acdS gene of PAC BNM0522 was amplified with sequencespecific forward (5’-ATGAACCTGCAACGATTC-3’) and reverse (5’- TCAGCCGTCTCGGAAGAT-3´) primers. PCR reactions were carried out in 20 μl reaction mixture containing 10 x buffer with 50 mmol.l-1 MgCl2, 0.5 μl; 10 mmol.l-1 DNTPs mixture, 1 μl; Taq DNA polymerase, 1 μl (Invitrogen); template, 10 ng; and water until completing the final volume. DNA samples were amplified on a DNA thermal cycler (PTC-100 TM Programmable Thermal Controller, MJ Research Inc., Canada). The PCR program consisted of an initial denaturation at 94ºC for 1 min followed by 35 cycles of 94ºC for 30 s, 67ºC for 45 s, and 72ºC for 45 s, with final extension of 5 min. Samples (10 μl) of PCR products were separated on 1.5% agarose in TAE buffer containing 0.5 μg of ethidium bromide per ml at 65 V for 1 h. The amplified PCR products were visualized in agarose gel with a UV transilluminator and photographed using the gel documentation system (E-Box VX2, Vilber Lourmat, France).
IAA production
The bacteria were grown at 33ºC with gentle shaking in TSB supplemented with tryptophan (25 mg. l-1) for 48 h. After that, 1-ml samples were withdrawn and IAA content was measured as described in Shoebitz et al. [20].
Nucleotide sequence accession number
The 16S rDNA sequence for strain PAC BNM 0522 was submitted to GenBank (accession number FJ851181) and compared with known sequences using the BLASTN PROGRAM (megablast) [21].
Antifungal activity of PAC BNM 0522
The antibiosis and inhibition of S. rolfsii sclerotial germination were studied according to Shoebitz et al. [20] using a 9-mm diameter agar plug containing S. rolfsii 7-day-old mycelium. Sclerotia were collected from 20-day-old fungal cultures and superficially disinfected by soaking them for 5 min in 15% sodium hypochlorite solution containing 0.1% Triton X-100, before two washes with sterile water. Fifteen sclerotia were placed in potato dextrose agar (PDA) medium and inoculated with 15 μl of 6 × 108 CFU ml-1 of each bacterial suspension. The assays were repeated three times.
Direct and indirect mechanisms of plant growth promotion
Seeds of tomato (cv Roma and Rio Grande) were superficially disinfected according to Ribaudo et al. [22] and sown in pots (90 mm x 90 mm) containing sand:perlite 3:1 v/v and planting two seeds in each and maintained in a Sanyo® MRL-350 culture chamber under 16/8 h light/dark, 25ºC, 80% relative humidity, and light intensity of 350 mE m-2 s-1 for 60 days. One set of plants was used to evaluate the plant growth promotion induced by PAC BNM0522 and another set was used to evaluate the induced resistance of tomato plants after pathogen challenge (ten pots were used in each treatment). The following plant parameters were measured on 20 plants from each replicate: shoot and root fresh weight, shoot height and main root length. ANOVA was used to test differences and Fisher´s Test was used for comparison of means. All statistical analyses were performed with InfoStat software (Universidad Nacional de Córdoba, Argentina). Statistical significance was set at P<0.05.
At 50 days after sowing (DAS), plants were inoculated with one sclerotium each, placed at the base of the stem. The evolution of the disease was evaluated by measuring the percentage of infected plants (incidence) or dead plants (mortality) in a time course experiment from 24 h to 10 days after inoculation with sclerotia. The experiments were repeated twice.
Ethylene production
Ethylene production in plants grown in the conditions described above was measured as described in Ribaudo et al. [22] and finally the weight of five fresh fragments from each plant was determined.
Analysis of gene expression involved in ethylene biosynthesis by PCR
For gene expression analysis, a pool of leaves from four plants was ground in liquid nitrogen and 100 mg of the powdered tissue was used for each experiment. Extraction and purification were performed using an RNAeasy plant MINIKIT (Quiagen), according to the manufacturer ´s instructions. First-strand cDNA was prepared using SuperScript II RNAse H-Reverse Transcriptase (Invitrogen) following the manufacturer´s specifications.
Oligonucleotide primers were designed based on sequences available at the database (http://www.ncbi.nlm.nih.gov/ and http:// solgenomics.net) and synthesized by Operon Biotechnologies, USA. The sequences of interest were amplified by PCR using the enzyme Taq DNA Polymerase Recombinant (Invitrogen). The amplification mixture contained 10 ng cDNA in 10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 100 μM deoxynucleotide triphosphates, 5U Taq DNA polymerase, and 2 μM of the specific primers. For Sl-ACS2 (GenBank: X59139.1), the primers used were Sl-ACS2 -F (5’CTGGTGGTGCCACTGGGGCT3’) and Sl-ACS2 -R (5’CTCTAAATCCTGGTAACCC3’) and PCR conditions were an initial step at 94ºC for 1 min, followed by 30 cycles of 94ºC for 30 s, 68ºC for 45 s, and 72ºC for 45 s and a final extension step at 72ºC for 4 min. For Sl-ACS3 (GenBank: NM 001247097.2), the primers used were Sl-ACS3 -F (5´CGGTCTCCCCGGTTTTCGCA3´) and Sl-ACS3 -R (5 ´GCGTTGGGAGCAGTGGACTCG3´), and the conditions for amplification were an initial step at 94ºC for 1 min, followed by 30 cycles of 94ºC for 30 s, 63ºC for 45 s and 72 for 60 s, and a final extension step at 72ºC for 5 min. For Sl-ACS5 (GenBank: ACS5: AF179246.1), the primers used were Sl-ACS5 -F (5 ´TGCTCGCTTCAGGTCTCGCA3’) and Sl-ACS5 -R (5´TGACCCAGCACCCGTCACGA3’). For Sl-ACS6 (GenBank: AF179249.1), the primers used were Sl-ACS6- F (5 ´CGCGAAGAGCTCGATGAGATTGT3’) and Sl-ACS6 -R (5 ´GCGTTGGGAGCAGTGGACTCG3´). For Sl-ACO1 (GenBank: HQ322499.1), the primers used were Sl-ACO -F (5 ´CCGCGCTCATACAGACGAGG3’) and Sl-ACO -R (5 ´AGGCTAATGACATTCGTGTCCCGT3’). The conditions for amplification for Sl-ACS 5 , SlACS 6 and SlACO1 were an initial step at 94ºC for 1 min, followed by 30 cycles of 94ºC for 30 s, 64ºC for 45 s and 72 for 60 s, and a final extension step at 72ºC for 5 min. For the constitutive gene Sl-UBI3 (GenBank: X58253.1), the primers used were Sl-UBI3 -F (5’CGAAGCCTCTGAACCTTTCC3’) and Sl-UBI3 -R (5’GGATTCCCCCAGACCAGCAG3’) and the PCR conditions were an initial step at 94ºC for 1 min, followed by 30 cycles of 94ºC for 30 s, 62ºC for 45 s, and 72ºC for 1 min and a final extension step at 72ºC for 5 min. The reaction products were resolved in 1% agarose gels. The gels were photographed using EBOX VX2 gel documentation system (Vilmert Lourmat, France).
Evaluation of endophytism
Plants were sampled at 60 DAS and the tissues (shoots and roots) were disinfected with chloramine T solution (1% w/v) for 15 min under gentle agitation followed by three washings with sterile water. The tissues were ground in a mortar and the number of CFU was determined by serial dilution plating on Cetrimide agar amended with tetracycline (30 μg/ml), and potato medium for PAC BNM0522 and A. brasilense, respectively [23].
Results
Bacterial identification
Molecular and biochemical assays were used to identify the strain PAC BNM0522. The biochemical profiling is summarized in Table 1.
| Test | PAC BNM0522 |
|---|---|
| Shape | Round rods |
| Gram stain | negative |
| Mobility | + |
| Colony color in cetrimide agar | green |
| Oxidase C | + |
| Catalase | + |
| N03 - reductase | + |
| Gelatin hydrolysis | + |
| Starch hydrolysis | + |
| Citrate Utilization | + |
| Urease activity | - |
| Use of sucrose in N-free medium | - |
| O/F glucose | +/- |
| O/F sacarose | -/- |
| O/F lactose | -/- |
| pigment production in P agar | + |
| pigment production in F agar | + |
| Growth at 4°C | - |
| Growth at 41°C | + |
| Growth in the presence of 4 % NaCl | + |
| Growth in the presence of 5 % NaCl | + |
| H2S production | - |
| Growth in pH from 5 to 10.5 | + |
| Optimum pH growth | 7.5 |
Table 1: Biochemical profiling of PAC BNM0522 strain.
In tests of the ability of PAC BNM0522 to grow under a range of temperature and pH, it grew up to pH 10.5 and 41ºC. PAC BNM0522 was slightly affected under salt stress. The bacterium grew well on plates containing different salt concentrations up to 5% NaCl (w/v). Regarding A. brasilense, its CFU were seriously reduced when subjected to an increased concentration of salt (it was incapable to grow at 5% NaCl w/v).
| Cell number (CFU.ml-1) | ||
|---|---|---|
| NaCl (% w/v) | A. brasilense | PAC BNM0522 |
| 0 | 3.25 × 1010 | 3.67 × 1010 |
| 0.8 | 1.65 × 1010 | 2.26 × 1010 |
| 1.7 | 5.45 × 105 | 1.8 × 109 |
| 3.5 | 1.02 × 102 | 5.84 × 108 |
| 5 | 0 | 2.34 × 105 |
Table 2: Effect of salinity on the bacterial cell number.
The antibiotic resistance test showed that PAC BNM0522 was resistant to the following antibiotics: 10U/ml penicillin, 5 μg/ml novobiocin, and 30 μg/ml tetracycline, but sensitive to 10 μg/ml gentamicin, 30 μg/ml neomycin, 25 μg/ml streptomycin and 1.0 mg/ml of kanamycin. The experiment was repeated three times with identical results.
Based on the RapID NF Plus system results, PAC BNM0522 was identified as Ralstonia pickettii (synonym Pseudomonas pickettii, Ralston et al. [24] and included strains designated as Pseudomonas pseudoalcaligenes bio.2 with “adequate identification” (99.71% probability level). When the search was limited to records matching P. pseudoalcaligenes, the BLAST analysis of the 16S rRNA gene showed that PAC BNM0522 was similar to P. pseudoalcaligenes X4 (reclassified as Burkholderia and, subsequently, as Ralstonia) with 99% similarity of the ribosomal 16S RNA gene sequence [24,25].
ACC metabolism of PAC BNM0522
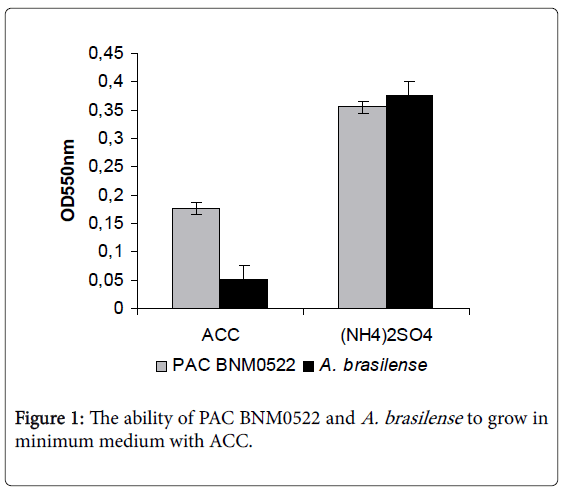
We evaluated the ability of PAC BNM0522 to use ACC as the sole nitrogen source. Figure 1 shows the ability of PAC BNM0522 and A. brasilense to grow in minimum medium with ACC. PAC BNM0522 was found to be positive for ACC deaminase production as indicated by its growth in the medium. In contrast, A. brasilense was unable to grow in this medium, suggesting that this strain does not produce the ACCD gene, as has been previously documented for some members of this genus [7].
Amplification of the ACCD gene (acdS )
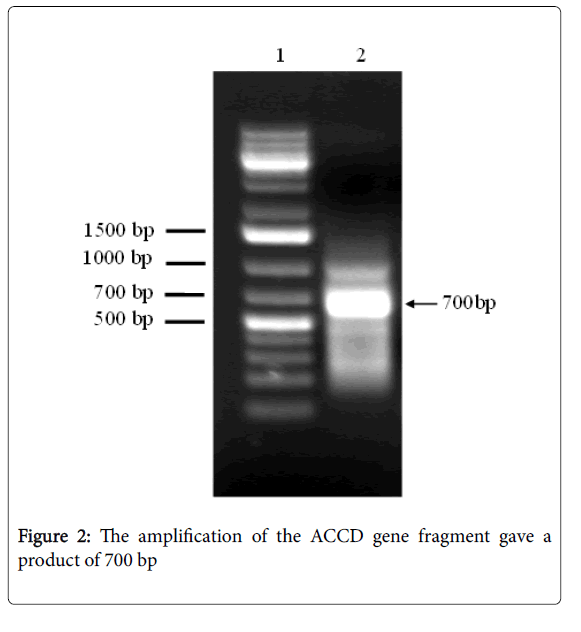
As expected, in the PCR experiments carried out to validate the induction of the ACCD gene from PAC BNM0522, using gene-specific primers, the amplification of the ACCD gene fragment gave a product of 700 bp (Figure 2). Thus, we confirmed that PAC BNM 0522 is an ACC deaminase-producing bacterium.
IAA production
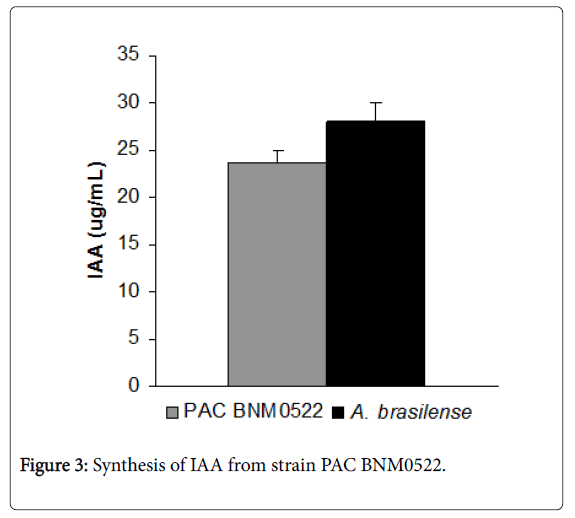
One of the most important aspects of PGPR is their ability to produce IAA, which improves the growth and physiology of crops. The strain PAC BNM0522 was able to synthesize IAA (Figure 3).
Like A. brasilense, PAC BNM0522 rendered a high level of IAA in the culture medium, considering the low amount of tryptophan in the culture medium.
Biocontrol activity of PAC BNM0522
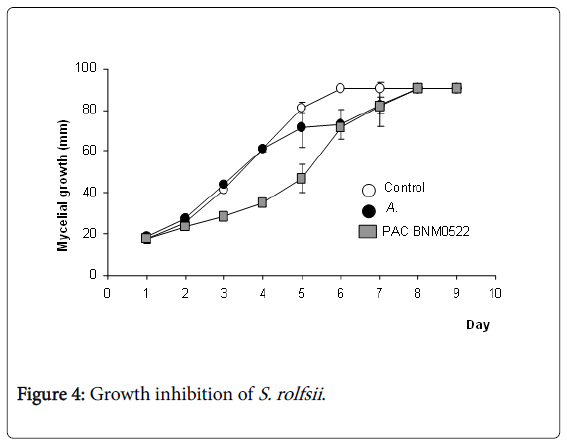
When S. rolfsii was grown together with PAC BNM0522 or A. brasilense in PDA for 9 days, the S. rolfsii mycelial growth was delayed from day 4, with 50% of growth inhibition, whereas A. brasilense was unable to inhibit fungal growth (Figure 4).
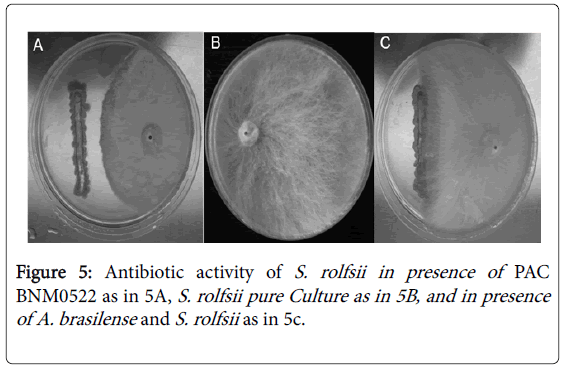
Antibiosis occurred during PAC BNM0522 interactions with S. rolfsii involving low-molecular-weight diffusible compounds or antibiotic production (Figure 5A), indicating an important antagonistic activity for this strain. In S. rolfsii pure cultures (Figure 5B), a layer of mycelia covered the surface of the agar, reaching the limits of the plate. Similar results were found when A. brasilense and S. rolfsii were evaluated (Figure 5C).
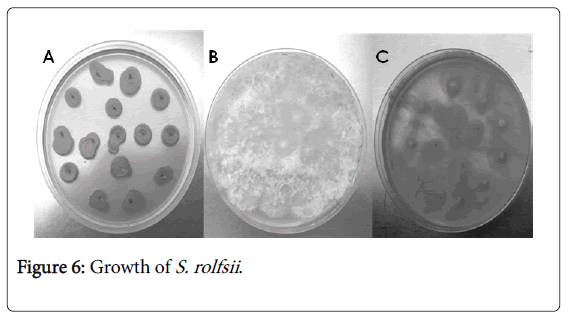
In the presence of PAC BNM0522, S. rolfsii sclerotia germination was 100% inhibited (Figure 6A), whereas in the presence of A. brasilense, germination was initiated but mycelial growth stopped after 72 h (Figure 6C). These results are very encouraging in the use of this strain as a biocontrol agent because the sclerotia are able to survive for many years in the soil.
Effect of PAC BNM0522 bacterization on disease evolution
With respect to S. rolfsii disease evolution, the incidence and mortality were evaluated for 10 days from the challenge (50 DAS) (Table 3).
| Treatment | Days after pathogen inoculation | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Control | 12.5 | 25 | 37.5 | 50 | 62.5 | 75 | 75 | 87.5 |
| PAC BNM0522 | 0* | 0* | 0* | 12.5 | 12.5 | 25 | 25 | 25 |
| A. brasilense | 0* | 0* | 0* | 25 | 37.5 | 50 | 62.5 | 75 |
| Mortality (%) | ||||||||
| Control | - | 12.5 | 25 | 25 | 37.5 | 50 | 50 | 62.5 |
| PAC BNM0522 | - | 0* | 0* | 0* | 0* | 25 | 25 | 25 |
| A. brasilense | - | 0* | 0* | 12.5 | 12.5 | 25 | 25 | 25 |
| The percentage of affected tomato plants was measured over 12 individuals during 10 days after pathogen inoculation. *indicates a statistically significant difference at a 5% level between treatments. | ||||||||
Table 3: Effect of PAC BNM0522 inoculation on the incidence and mortality rates in cv Roma plants challenged with Sclerotium rolfsii.
The incidence rate in control plants was about 87% at 10 days after Sclerotia infection, whereas that in plants whose seeds had been inoculated with PAC BNM0522 was 25% (62% less incidence than the control plants). Mortality of control plants was about 62%, whereas that of inoculated plants was only about 25% (Table 3). On the other hand, the incidence rate of plants inoculated with A. brasilense was 75%, whereas mortality was 25%.
Effect of PAC BNM0522 inoculation on endogenous levels of ethylene
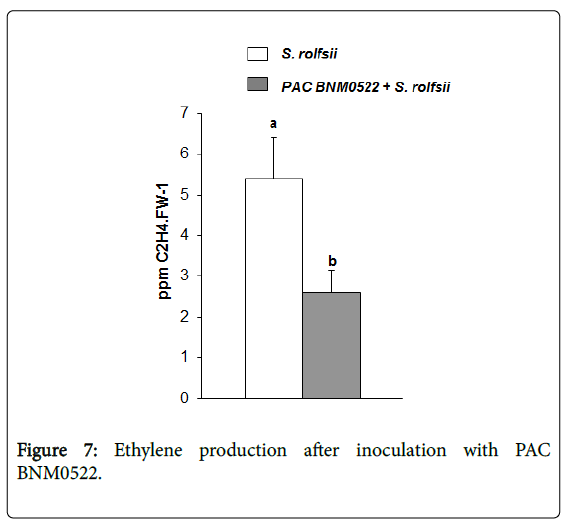
Apart from being a hormone related with defense responses of plants to pathogen attack, ethylene can also enhance disease, depending on the plant/pathogen combination. Plants treated with strains containing ACCD are more resistant to the damage caused by the ethylene synthesized as a consequence of the presence of a pathogen [26]. To evaluate the probable bacterial mechanism of action on ethylene production, the endogenous production of ethylene was measured at 48 h after pathogen inoculation. In seedlings previously inoculated with PAC BNM0522, ethylene production increased rapidly 48 h after the challenge with S. rolfsii , and then decreased drastically (50% lower) (Figure 7).
Analysis of the expression of genes involved in the ethylene pathway by PCR
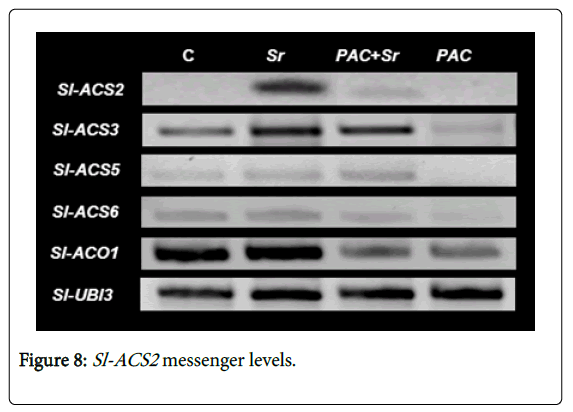
Among the members of the multigene family coding for ACS in tomato, Sl-ACS2 , Sl-ACS5 and Sl-ACS6 are strongly induced by a yeast elicitor [27]. To assess whether the increase in ethylene production in infected plants was correlated with the expression of Sl-ACS isoforms, the accumulation of Sl-ACS2 , Sl-ACS3 , Sl-ACS5 , Sl-ACS6 and Sl- ACO1 transcripts was evaluated at 24 h post-pathogen inoculation. Sl-ACS2 messenger levels appeared strongly upregulated in infected plants and down-regulated in PAC BNM0522-primed plants (Figure 8). The relative expression of Sl-ACS3 , Sl-ACS5 and Sl-ACS6 appeared nearly without changes in infected plants and undetectable or greatly diminished in PAC BNM0522-inoculated plants. The Sl-ACO1 messenger levels were significantly decreased in PAC BNM0522- inoculated plants challenged with S. rolfsii as compared to those infected with S. rolfsii alone. Also the Sl-ACO1 gene appeared strongly sub-expressed in PAC BNM0522-inoculated plants. This may be possible because ACC is being used by the concomitant reaction catalyzed by bacterial ACCD.
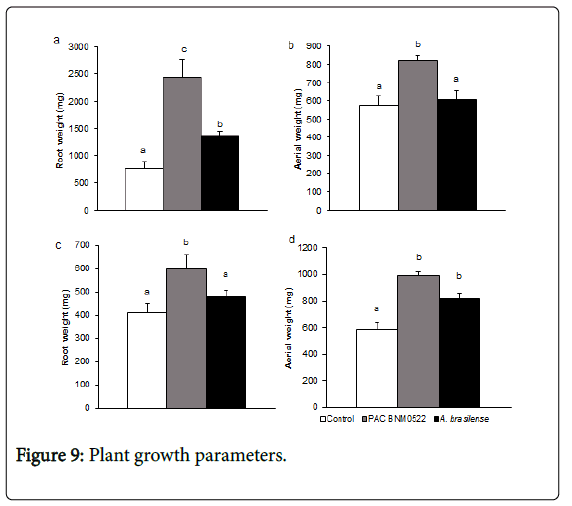
Tomato growth promotion by PAC BNM0522
Plant growth parameters (fresh root and shoot weight) were recorded after 60 DAS on VF36 and Roma cultivar tomato seedlings. Inoculation with ACCD-containing PAC BNM0522 greatly increased root weight (5.0 fold as compared to non-inoculated controls), whereas A. brasilense increased root weight 2.0 fold as compared to VF36 cultivar tomato control plants (Figure 9A).
Data revealed that both rhizobacteria were effective in increasing root weight. In contrast, shoot weight in PAC BNM0522-inoculated plants was 45% higher than that in non-inoculated controls. No differences were observed in A. brasilense -inoculated plants (Figure 9B).
PAC BNM0522 was also screened for its growth promoting activity in tomato cv Roma. Root and shoot fresh weight in PAC BNM0522- inoculated plants was 50% and 67% higher respectively, whereas shoot fresh weight in A. brasilense -inoculated plants was 33% higher (Figure 9D).
Evaluation of endophytism
PAC BNM0522 was isolated from surface-disinfected root and shoot tissues of inoculated plants. The number of bacteria was 2.5 105 CFU/g FW and 5.7 105 CFU/g FW in cv VF36 roots and shoots respectively for PAC BNM0522 and 2.7 103 CFU/g FW and 2.1 104 CFU/ g FW for A. brasilense -inoculated plants. With respect to cv Roma, the CFU were 2.3 104 CFU/g FW and 6.3 104 CFU/g FW in roots and shoots for PAC BNM0522 and 2.7 103 CFU/g FW and 2.1 104 CFU/ g FW for A. brasilense. Thereby, PAC BNM0522 was able to colonize and persist inside tissues, without causing damage to tomato plants.
Discussion
Among soil bacteria, the genus Pseudomonas is one of the most diverse and widespread in natural environments and many species display plant growth promotion [28]. In the present study, the identification of PAC BNM0522 allowed us to classify it as Pseudomonas and, according with the biochemical profiling and 16S rDNA sequence, it seemed to correspond to P. pseudoalcaligenes. Bacteria are able to adapt to a certain range of changes in external osmolarity by the accumulation of osmoprotective compounds. In the present study, PAC BNM0522 showed osmotolerance; it grew well from 0.8 to 5% NaCl. It has been reported that certain salt-tolerant strains of pseudomonads such as P. pseudoalcaligenes, P. alcaligenes, and Stenotrophomonas are able to grow in the rhizosphere under salt conditions [29,30]. We also found a positive effect of PAC BNM0522 on tomato seed germination and emergence in salt medium, probably attributed to the fact that PAC BNM0522 contains ACCD [31] (Ribaudo, personal communication).
Plants exposed to biotic or abiotic stress conditions produce ethylene from its precursor ACC. The role of ethylene in plant pathogen interactions is complex. Ethylene perception is often required for basal resistance, but the production of ethylene upon infection can aggravate symptom development [4]. Many PGPR can hydrolyze ACC, thereby lowering the levels of ethylene. The method to screen the bacterium ability to use ACC as a sole nitrogen source allowed us to select PAC BNM0522 as an ACCD-producing bacterium and further investigate the relative expression of its ACCD gene (acdS ). A single band with 700 bp confirmed the presence of mRNA acdS in this bacterium grown in TSB amended with ACC [32]. We next examined the biocontrol ability of PAC BNM0522 against root pathogens in further detail and demonstrated that tomato was protected against pathogenic soil fungi. In in vitro assays, through a dual culture technique, this strain showed antifungal activity against mycelia growth of S. rolfsii, which was evident by a decreased mycelial border. In addition, we observed that PAC BNM0522 efficiently inhibited in vitro sclerotia germination (100%).
When we tested the antagonistic activity of PAC BNM0522 against S. rolfsii on tomato, we found that the bacterium, confined to the plant root system, was still protective when the sclerotia were deposited at the base of the stem. Since in this case the rhizobacteria and the fungus were never in direct contact with each other in the plant, the protective effect had to be plant-mediated. Thus, we evaluated the induction of plant defense in tomato plants. The role of ethylene as a mediator in the response of plants to pathogen attack has been addressed in numerous studies [33-35]. The positive role of ethylene in disease development in tomato plants has been previously reported for infections with the necrotrophic fungi S. rolfsii [36]. In the present study, the presence of the fungus at the base of the stem seriously compromised the health of the plants and promoted a marked systemic increase in ethylene production in plant shoots. The ability of PAC BNM0522 to ameliorate the damage in tomato seedlings was evident by the incidence and mortality rate (25% at 10 days after sclerotia inoculation and 87 and 62% respectively for control plants). The significant decrease in ethylene content in primed plants challenged with S. rolfsii (50% lower) is in concordance with the low gene expression of Sl-ACS2 and Sl-ACO1 . The biocontrol effect on necrotrophic fungus through a decrease in ACC content via ACCD enzymatic removal has been demonstrated in other PGPR/plant associations [35,36]. Barnawal et al. [36] showed decreased ACO and ACS activities and decreased ACC content (60% lower) in Pisum sativum PGPR-inoculated plants We found that among the ACS isoforms expected to be up-regulated by the pathogen challenge, the expression of Sl-ACS3 , Sl-ACS5 and Sl-ACS6 was virtually unchanged at 24 h after infection with S. rolfsii. In contrast, Sl-ACS2 appeared down-regulated in primed plants. All Sl-ACS isoforms were strongly suppressed in the PAC BNM0522-inoculated plants and Sl-ACO1 was negatively regulated by the presence of the bacterium. The fact that the Sl-ACS3 , Sl-ACS5 and Sl-ACS6 levels were unchanged after challenging with S. rolfsii suggests that they could be some of the ACS isoforms not responsible for the increase in ethylene level upon infection or that their expression is not an early event in the plant response to the pathogen. These results are in concordance with those of Dixit et al. [36], who found that S. rolfsii -treated plants recorded an increase in ACS and ACO gene expression (5.02- and 3.39-fold higher expression respectively) and that inoculation of ACC deaminaseproducing bacteria (B-30488 strain) reduced the expression of these two genes even in S. rolfsii -treated plants. These results allow us to conclude that ethylene promotes disease development in tomato plants infected with S. rolfsii and that the inhibition of ethylene biosynthesis in primed plants correlates with and depends -at least largely- on the concomitant down-regulation of the Sl-ACS2 and Sl-ACO1 genes. To better understand the mechanisms involved in the response to infection, it would be informative to study whether or not the expression of other Sl-ACS and Sl-ACO isoforms - which could contribute to the overall synthesis of ethylene- is affected by the infection and to investigate how commonly this strategy is used in other disease-causing pathogens whose development depends on the production of ethylene. Barnawal et al. [35-36] detected reduced ACS activity simultaneously with reduced ACC content in PGPR-inoculated plants. Our results are in agreement with this. A lower Sl-ACS mRNA content in PAC BNM0522-inoculated plants may be responsible for a decrease in ACC content in inoculated plants and thus for a diminished ethylene level. Both the ACCD activity and low expression of ACO messenger found in inoculated plants may be responsible for the putative plant protection under pathogen stress, alleviating ethylene-induced damage.
The ability to synthesize IAA is widespread among soil- and plantassociated bacteria. In culture medium supplemented with the lowest amount of tryptophan, PAC BNM0522 synthesized higher level of IAA. Patten and Glick [35] studied bacterial strains with and without the ability to produce IAA and showed that the IAA-producing strains increased canola root length. Our results indicate that inoculation of tomato seedlings with PAC BNM0522 promoted the development of plants, as revealed by the increase in shoot and root biomass. The ethylene content in the plant would be the result of the balance between its synthesis, stimulated by a higher level of IAA promoted by the PGPR, and its sequestration through the activity of the bacterial ACCD [36]. Because A. brasilense lacks ACCD, the A. brasilense - inoculated tomato plants showed an increase in ethylene production [22], whereas plants inoculated with PAC BNM0522 showed a reduced level of ethylene due to the presence of ACCD and the downregulation of some Sl-ACS isoforms related to pathogenesis. It would be of interest to determine whether other members of the ACS and ACO gene families are expressed in response to PGPR treatment.
The presence of PAC BNM0522, evaluated by root and shoot bacterial colonization, and the absence of damage in plant tissues allowed us to state that PAC BNM0522 is a beneficial rather than a deleterious endophyte for the plant. In areas seriously affected by the presence of the highly resistant soil-borne fungus S. rolfsii, PAC BNM0522 may contribute to relieving disease severity.
Acknowledgements
The authors are grateful to M. Mitidieri for providing S. rolfsii and J. Döbereiner for providing us with the Azospirillum brasilense FT326. V. Feuring is acknowledged for assistance with ethylene measurements.
Conflict of Interest
All authors have declared no financial/commercial conflicts of interest.
References
- Bari R, Jones JD (2009) Role of plant hormones in plant defence responses.Plant MolBiol 69: 473-488.
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity.Nat ChemBiol 5: 308-316.
- van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants.Trends Plant Sci 11: 184-191.
- Lund ST, Stall RE, Klee HJ (1998) Ethylene regulates the susceptible response to pathogen infection in tomato.Plant Cell 10: 371-382.
- Iqbal N, Trivellini A, Masood A, Ferrante A, Khan NA (2013) Current understanding on ethylene signaling in plants: the influence of nutrient availability.Plant PhysiolBiochem 73: 128-138.
- Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant MolBiol 44: 283–307.
- Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world.Microbiol Res 169: 30-39.
- Jha Y, Subramanian R (2014) Characterization of root-associated bacteria from paddy and its growth- promotion efficacy. Biotech 4: 325-330.
- Gontia-Mishra I, Sasidharan S, Tiwari S (2014) Recent developments in use of 1-aminocyclopropane-1-carboxylate (ACC) deaminase for conferring tolerance to biotic and abiotic stress.BiotechnolLett 36: 889-898.
- Glick BR, Jacobson GB, Schwarze MK, Pastenak JJ (1994) 1-aminocyclopropano 1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacteriumPseudomonas putida GR 12-2 do not stimulate canola root elongation. Can J Microbiol 40: 911-915.
- Belimov AA, Safronova VI, Mimura T (2002) Response of spring rape (Brassica napus var. oleifera L.) to inoculation with plant growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase depends on nutrient status of the plant.Can J Microbiol 48: 189-199.
- Singh UP, Sarma BK, Singh DP, Bahadur A (2002) Studies on exudate - depleted sclerotial development in Sclerotiumrolfsii and the effect of oxalic acid, sclerotial exudate, and culture filtrate on phenolic acid induction in chickpea (Cicerarietium). Can J Microbiol 48: 443-448.
- Sharma R, Arunabh J, Dhaker RC (2012) A brief review on mechanism of Trichoderma fungus use as biological control agents. Int J Innov Bio-Sci 2:200-210.
- Dixit R, Agrawal L, Gupta S, Kumar M, Yadav S, et al. (2016) Southern blight disease of tomato control by 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing Paenibacilluslentimorbus B-30488. Plant Signaling Behavior 11: e1113363.
- Nico M, Ribaudo CM, Gori JI, Cantore ML, Curá, JA (2012) Uptake of phosphate and promotion of vegetative growth in glucose-exuding rice plants (Oryza sativa) inoculated with plant growth-promoting bacteria. Applied Soil Ecol 6: 190-195.
- Levine M, Carpenter DC (1923) Gelatin Liquefaction by Bacteria.J Bacteriol 8: 297-306.
- Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen.J Bacteriol 75: 592-603.
- Schoebitz M, Ribaudo CM, Pardo MA, CantoreML,Ciampi L, et al. (2009) Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Loliumperennerhizosphere. Soil BiolBiochem 41: 1768-1774.
- Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences.J ComputBiol 7: 203-214.
- Ribaudo CM, Krumpholz EM, Cassán FD, Bottini R, Cantore ML et al. (2006) Azospirillum sp. Promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 25: 175–185.
- Dobereiner J, Marriel JE, Nery M (1976) Ecological distribution of SpirillumlipoferumBeijerinck. Can J Microbiol 22: 1464-1473.
- Ralston E, Palleroni NJ, Doudoroff M (1973) Pseudomonas picketti, a new species of clinical origin related to Pseudomonas solanacearum. Int J SystBacteriol 23: 15-19.
- HUGH R, RYSCHENKOW E (1961) Pseudomonas maltophilia, an alcaligenes-like species.J Gen Microbiol 26: 123-132.
- Toklikishvili N, Dandurishvili N, Vainstein A, Tediashvili et al. (2010) ACC deaminase-producing bacteria inhibit crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol 59: 1023-1030.
- Oetiker JH, Olson DC, Yin Shiu O, Fa Yang S (1997) Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersiconesculentum). Plant MolBiol 34: 275-286.
- Valverde C, Agaras B (2013) Pseudomonas in agricultural soils and rhizosphere. From molecules to communities. In: García de Salamone IE, Vázquez S, Penna C, Cassán F (edn.) Rizosfera, Biodiversidad y AgriculturaSustentable. AAM, Buenos Aires.
- Alavi P, Starcher M, Zachow C, Muller,et al. (2013) Root-microbe systems: the effect and mode of interaction of Stress Protecting Agent (SPA) Stenotrophomonasrhizophila DSM14405 T. Front Plant Sci 141: 152-160.
- Mayak S, Tirosh T, Glick BR (2004) Plant growth promoting bacteria confers resistance in tomato plants to salt stress. Plant PhysiolBiochem 42: 565-572.
- Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacterprotophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisumsativum.J Plant Physiol 171: 884-894.
- Sheehy RE, Honma M, Yamada M, Sasaki T, Martineau B, et al. (1991) Isolation, sequence, and expression in Escherichia coli of the Pseudomonas sp. strain ACP improving growth and yield of wheat (Triticumaestivum L). J MicrobiolBiotechnol 17: 1300-1307.
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology.(2nd Edn.), Academic Press, San Diego.
- Knoester M, Van Loon LC, Van Den Heuvel J, Hennig J, Bol JF, et al. (1998) Ethylene insensitive tobacco lacks nonhost resistance against soil-borne fungi. ProcNatlAcadSci 95: 1933-1937.
- Knoester M, Pieterse CMJ, Bol JF, Van Loon LC (1999) Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant-Microbe Interact 12: 720–727.
- Dixit R, Agrawal L, Gupta S, Kumar M, Yadav S, et al. (2016) Southern blight disease of tomato control by 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing Paenibacilluslentimorbus B-30488. Plant Signaling Behavior 11: e1113363.
- Patten CL, Glick BR (2002) Role of Pseudomonas putidaindoleacetic acid in development of the host plant root system.Appl Environ Microbiol 68: 3795-3801.
- Gamalero E, Glick BR (2015) Bacterial Modulation of Plant Ethylene Levels.Plant Physiol 169: 13-22.
Copyright: © 2016 Ribaudo CM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.