Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Review Article - (2024)Volume 10, Issue 1
Cardiotoxicity is an unwanted side-effect arising from different medications. The term describes a diminution of heart function, affecting especially left ventricular ejection fraction, which can progress to heart failure. Pediatric oncology patients treated with drugs of the anthracycline group, e.g., doxorubicin, can evolve to heart failure patients in adult age. Nuclear medicine imaging of fatty acid oxidation and glycolysis accurately describes normal and altered heart metabolism. The most common finding is a state of cardiac glycolysis, which is reflected by increased uptake of 18F-desoxyglucose (18F-FDG) at a time when fatty acid oxidation is decreased. Glycolysis has recently been recognized as being the result of coenzyme Q10 deficiency as part of adaptation to hypoxia. Low LEVF correlates with impaired fatty acid oxidation. This deficiency can arise from chemotherapy and radiation and can be corrected by supplementation. Drugs like enalapril can improve fatty acid utilization. We will review relevant nuclear medicine imaging studies as well as fundamental biochemical data that demonstrate this pathogenetic path.
Coenzyme Q10; Glycolysis; Hypoxia; 18Fluoro-desoxyglucose; 18F-FDG; Positron emission tomography; BMIPP; Fatty acid oxidation; Baseline diagnosis; Pediatric oncology; Post-partum cardiomyopathy.
Pediatric oncology and cardiotoxicity
The historical aspects of clinical pediatric oncology as a medical specialty were described by Pearson in who traced its begin to medical care of hematological diseases [1]. In cardiooncology was proposed as a new field in medicine [2].
These contributions have led to clinical work from the standpoint of oncologists and cardiologists in relation to changes of heart function in the sense of cardiotoxicity. Unfortunately, despite decades of clinical research, neither a clinically useful biochemical explanation of cardiotoxicity nor a therapeutic approach have been produced.
Cardiotoxicity is understood as a clinical entity related to diminished heart function, which Beutner first described in relation with the use of injectable anesthetics [3]. Many pharmaceutical substances were recognized as cardiotoxic in the following decades [4]. These drugs belong to different pharmacological groups and have been enumerated in a publication by the American Heart Association [5]. Drugs that are effective in anti-cancer treatments can affect heart function in different ways [6-8], leading to cardiac damage and heart failure [9]. Cardiotoxic anti-cancer drugs include anthracyclines, alkylating agents, topoisomerase inhibitors, taxanes, and others [10].
After tumors have been treated successfully, childhood cancer survivors face the risk of developing chronic heart diseases in adult age. Oeffinger et al., have called this a chronic health condition where the most common diseases are second cancers, cardiovascular disease, renal disease, musculoskeletal problems, and endocrine diseases [11]. Robison and Hudson described a complex health situation for childhood cancer survivors arising from the use of radiation, chemotherapy, or surgery (Figure 1) [12].
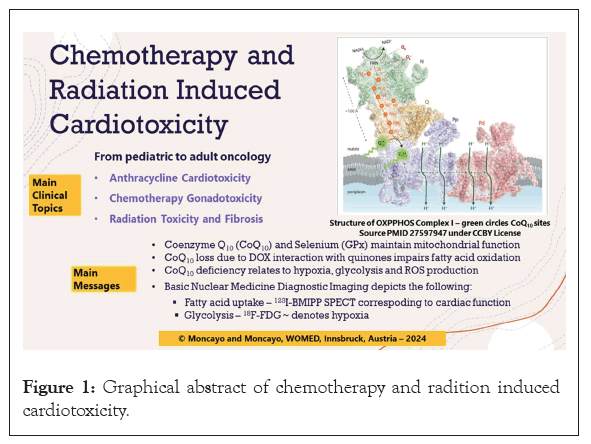
Figure 1: Graphical abstract of chemotherapy and radition induced cardiotoxicity.
The appearance of disease seems to have a variable latency time. Tumor types at the primary diagnosis can be leukemia, lymphoma, central nervous system tumors, Wilms tumor, neuroblastoma, rhabdomyosarcoma, and bone cancer [13]. Another medical situation associated with cardiotoxicity in adult life is hematopoietic stem cell transplantation [14].
A clinical description of Hodgkin’s lymphoma, published by Brice et al., de Kerviler, and Friedberg, pointed out that many patients died because of late toxic effects of the treatment [15]. Maraldo et al. approached the topic of cardiovascular disease after therapy for Hodgkin’s lymphoma in using a questionnaire approach. The authors found 19% of cases reporting ischemic heart disease, 12% with congestive heart failure, as well as arrhythmia and valvular disease [16]. Bergom et al., analyzed radiation-induced cardiac changes. They proposed that the goal of screening should be the detection of cardiac injury before it becomes clinically evident, expecting that dosimetry and advanced imaging would answer this problem [17].
In cardiotoxicity was described as a series of changes in the heart including cell injury, deformation, and left ventricular dysfunction (Figure 1) [18]. López-Sendón and coworkers described an incidence of cardiotoxicity of 37.5% among 865 patients from a prospective study [19]. The relevance of evaluating left ventricular function was stressed by Tsiouris et al., [20] as well as by Gavila et al. [21]. The authors pointed out the need to identify affected patients using cardiological diagnostic criteria such as the periodic evaluation of the left ventricular ejection fraction. The authors praised their own work as the first one to produce statements related to prevention, assessment, and monitoring of cardiac toxicity after chemotherapy. The publication, unfortunately, did not suggest any therapeutic procedure to achieve these goals. Belger et al. and coworkers analyzed risk factors in relation to cardiotoxicity and concluded inconclusively that “a better understanding” would be desirable [22]. The same applies to surveillance programs based on epidemiological data [23,24]. Epidemiology alone, cannot deliver treatment options and is disappointing for patients.
An important aspect of heart physiology to be kept in mind is the balance between energy requirement and energy supply. Brown and coworkers described this issue and discussed a potential role of mitochondrial dysfunction in heart disease expecting that further research would fulfill therapeutic goals (Figure 2) [25].
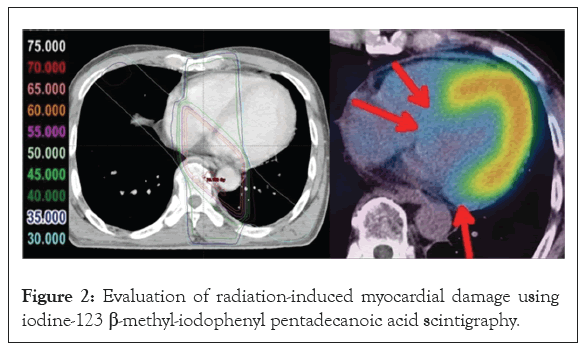
Figure 2: Evaluation of radiation-induced myocardial damage using iodine-123 β-methyl-iodophenyl pentadecanoic acid scintigraphy.
In this review we will deal with cardiac side effects of anthracyclines and those due to external radiation therapy. We will focus on research and development work of daunomycin (adriamycin, doxorubicin) since this group of substances is relevant for treating breast cancer and other solid tumors. Besides cardiotoxicity we will also briefly discuss postpartum cardiomyopathy and gonadotoxicity.
Our emphasis will be on metabolic aspects such as fatty acid oxidation and glycolysis, two basic processes related to heart function. We will construct this description based on nuclear medicine diagnostic imaging together with biochemical parameters that are relevant to clinical practice.
Searching the early literature for biochemical elements related to heart failure, we identified a series of publications that related coenzyme Q10 (CoQ10) deficiency to heart disease. For this reason we have chosen the publication by Folkers et al. as a central reference for our review [26].
Pharmaceutical development of natural anti-tumor substances
A historical account of the early research of new anti-tumor substances in Italy was published by Cassinelli [27]. He described how in May 1960, a fruitful connection had been established by Dr. Bertini, CEO of Farmitalia, and Prof. Bucalossi, director of the Istituto Nazionale dei Tumori, to start research on new natural anti-tumor substances. Adriamycin was isolated as an antibiotic substance in the Farmitalia Research Laboratories working with a mutant strain Streptomyces peucetius bacterium (Streptomyces peucetius caesius). Dott reported the discovery of daunomycin [28]. In the same year, Dubost et al., reported the discovery of rubidomycin, a substance related to daunomycin [29]. A description of the therapeutical use of daunomycin followed [30]. Rcamone et al. reported the production of the new anti-tumor substance 14-hydroxydaunomycicn (Adriamycin) [31]. Phase I and preliminary Phase II studies with adriamycin were published [32]. The patent, describing the anti-tumoral activity of the compound, was granted in 1971 to Farmitalia Carlo Erba Spa [33]. At that time, no cardiac side effects were mentioned.
Bonadonna et al., described indeed ECG changes after treatment with adriamycin, i.e., doxorubicin [34]. Previously, Tan et al. had also reported cardiotoxicity after the administration of daunomycin [35].
In 1981, di Marco, et al. published a historical review on the discovery of daunorubicin [36]. Readers interested in further historical aspects of these developments can also consult the publication by Waksman and Woodruf on the discovery of Actinomyces antibioticus [37]. An important notion to be kept in mind is that actinomycin has a quinone structure as described by Dalgliesh and Todd [38]. Additional studies from the early past century had evaluated quinones concerning their structure and biochemistry and their role as disinfectants and antibiotics [39-43].
In the 1970s, scientific data clearly relating adriamycin to mitochondrial function appeared. Iwamoto Hansen, Porter, and Folkers working with beef heart mitochondria, described that adriamycin had an inhibitory action on cell respiration by affecting CoQ10 enzymes related to the electron transfer processes [44]. The rationale for their study was based on the quinoid and hydroquinoid structures of adriamycin and daunorubicin. In their conclusions, they described this inhibition of CoQ10 by adriamycin. Kishi et al. demonstrated a beneficial effect of CoQ10 supplementation for preventing adriamycin-induced inhibition of myocardial mitochondria affecting the NADH-oxidase and the succinoxidase systems. A molar ratio of 3:1 of CoQ10 to adriamycin prevented this inhibition [45]. An additional mechanism of cardiotoxicity by doxorubicin is the alteration of fatty acid oxidation and interference of carnitine palmitoyltransferase [46,47].
In an experimental setting with male Sprague-Dawley rats, weighing 180-200 g, Folkers et al. showed in that the administration of CoQ10 at a dose of 1 mg/ml/kg resulted in "rescue" of electrocardiographic abnormalities caused by adriamycin cardiotoxicity [48]. These experiments showed clearly a relation of the drug to CoQ10 and placed CoQ10 in the category of a therapeutical option.
The beneficial effects of CoQ10 or α-tocopherol we shown by Lubawy, Whaley, and Hurley in an experimental setting in [49]. Ohhara, Kanaide, and Nakamura resented data on the protection against cardiotoxicity due to adriamycin achieved by administering CoQ10. This effect was associated with a higher cardiac contractile tension and higher ATP stores [50]. Takimoto et al., described a protective effect of CoQ10 on the heart after the combined use of 500 rad irradiation with 60Co plus a mixture of adriamycin, cyclophosphamide and 5-FU can lead to cardiac toxicity [51]. Saltiel and Mcguire followed a similar line of thought mentioning the use of CoQ10, selenium sulfide, or α-tocopherol, among others, to prevent cardiotoxicity [52]. Tsubaki et al. described a preventive effect of CoQ10 administration on alterations of the ECG in patients treated either with adriamycin or daunorubicin [53].
Davies and Doroshow identified Complex I of mitochondrial OXPHOS as an essential metabolic site for doxorubicin [54]. An accompanying study showed that cardiac damage through doxorubicin was due to the production of a hydroxyl radical [55]. Following this line of research, Schimmel et al., illustrated oxidative stress mechanisms arising from anthracyclines related to their quinone structure (Figure 2) [56]. A recent 2020 study on cardiotoxicity and doxorubicin involving respiratory Complex I and mitochondria was published by Wallace, Sardão, and Oliveira [57].
An elegant experiment by Sarvazyan in delivered imaging data that demonstrated oxidation-sensitive fluorescence in isolated cardiac myofibers after administration of 160 μm of doxorubicin. They showed that an oxidative mechanism was seen already 20 minutes after exposure [58]. Newer data from Doroshow complemented these observations by demonstrating that quinones and anthracycline antibiotics can stimulate oxygen radical production in cardiac cells [59]. Conklin reported an alteration of mitochondrial function as the side effect of anthracyclines and proposed using CoQ10 to prevent these changes [60]. Recently, Botehlho et al., showed protective effects of CoQ10 in an experimental model of doxorubicin-induced cardiotoxicity in [61].
While these valuable studies indeed show beneficial cardiac effects of CoQ10 administration, they do not supply a biochemical explanation of the underlying situation (Figure 2) [12]. This can also be seen in a proposed protocol for early detection of subclinical left ventricular dysfunction published by Caspi and Aronson (Figure 2) [62].
Investigating cardiotoxicity in pediatric oncology
Lefrak, Pitha, Rosenheim, and Gottlieb published an analysis of clinical and pathological characteristics found in patients who presented cardiotoxicity due to adriamycin [63]. Their study included 399 patients who presented advanced carcinomas. They observed cardiotoxic effects such as transient changes in the ECG in 45 cases and severe congestive heart failure in 11. Eight patients with heart failure died because of cardiac decompensation. Cardiac involvement was suggested by diminution of QRS voltage, ventricular failure and cardiac dilatation, and lack of response to inotropic drugs. Postmortem studies showed a decrease in cardiomyocytes, loss of contractile elements, mitochondrial swelling, and intramitochondrial dense inclusion bodies. Electron microscopy studies showed alterations in mitochondria, including swelling and absence or degeneration of cristae (Figure 3) [63]. Since a therapeutic approach to this problem was not available at that time, they simply suggested to limit the total dose to less than 550 mg/m².
Figure 3: 18F-FDG cardiac PET/CT images before RT from beagles treated with two pre-scan preparations.
An early diagnostic approach to adriamycin cardiotoxicity and irradiation was the endomyocardial biopsy, as reported by Billingham [64]. Ulmer, Ludwig, and Geiger presented the assessment of systolic time intervals to detect cardiotoxicity [65]. Potential benefits and limitations in the use of M-mode echocardiography for the diagnosis of doxorubicin cardiotoxicity were presented by Markiewicz et al. in [66]. Ritchie et al. advocated radionuclide angiography as a basis and follow-up examination when anthracyclines were administered [67]. Lahtinen et al., introduced radionuclide ventriculography and found the method better than echocardiography and systolic time intervals [68]. Lewis et al. described the application of echocardiography [69]. Agarwala et al., described multiple gated acquisition scans as an investigation method [70]. A recent publication by Leerink et al. described changes of ejection fraction values in cases of cardiotoxicity. Surveillance intensity was adapted to the ejection fraction values [71].
Shulkin et al., described the use of Nuclear Medicine diagnostic imaging using 18F-fluorodeoxyglucose positron emission tomography (18F-FDG) [72]. In 2007 an updated version of this topic appeared [73]. Two Figures in this publication showed cardiac uptake: (Figure 3) shows the initial staging for a patient with an embryonal sarcoma; a similar pattern in follow-up examinations in a young patient with recurrent neuroblastoma. The authors did not explain the meaning of these cardiac images.
Cardiotoxicity due to irradiation
Reports on the toxic effects or irradiation on the heart have appeared since the late 1940s. Vince described the radiation dose administered to children with congenital heart disease during X-ray examinations. Over time, between 1931 and 1948, the recommended amount had been reduced as a measure to prevent side effects of radiation such as shortening of life span, increased incidence of malignant tumors, and premature aging [74].
The mechanistic background of radiation-induced cardiac damage has been characterized by newer studies [75]. Marks et al. described the development of cardiac perfusion defects following radiotherapy for left-sided breast cancer. The incidence of these changes showed an increase over time [76]. Boerma and Hauer-Jensen described the evidence on radiation-induced heart disease stressing the appearance of capillary changes, coronary artery disease, and fibrosis [77]. Insight into clinical aspects of radiation-induced cardiotoxicity has been summarized by Yang et al. [78].
Tapio has summarized experimental and clinical data on radiation-induced changes, mentioning biochemical mechanisms such as alterations in fatty acid oxidation and of PPARα signaling (Figure 3) [79]. Azimzadeh et al. showed that activation of PPARα by fenofibrate administration improved lipid metabolism and mitochondrial respiration [80]. Turunen et al. showed that PPARα exerted a positive influence on the biosynthesis of ubiquinone [81], thus indicating once more the central role of CoQ10. An additional biochemical alteration following irradiation is the decrease of selenium content in the heart, liver, and muscle [82].
The use of nuclear medicine diagnostic imaging to visualize cardiac metabolism and cardiotoxicity
The following paragraphs will discuss the use of radionuclide imaging to demonstrate fatty acid uptake based on 123I-beta-methyl-iodophenylpentadecanoic acid (BMIPP). Fatty acid uptake and oxidation is the primary source of energy for the heart. Publications dedicated to described the changes associated with cardiotoxicity have eluded the use of nuclear medicine procedures.
Knapp et al. introduced the BMIPP tracer into clinical practice in [83]. Ogata reported experimental data suggesting that adriamycin induced mitochondrial function impairment by decreasing myocardial uptake of BMIPP in rats, while heart perfusion evaluated with 201Tl, was uneventful compared to the control group [84]. The author had administered 4 mg/kg of adriamycin intraperitoneally for six days. The main changes observed in light and electron microscopy were related to changes in the mitochondria. Myofibrils and the nucleus were only minimally changed.
Wakasugi evaluated myocardial metabolism using two radionuclide imaging techniques BMIPP and 18F-FDG. They were applied in an experimental model with male Wistar rats where the animals had been treated with 2 mg/kg of adriamycin once weekly for 6-10 weeks. A radionuclide imaging evaluation was done two weeks after completing the treatment. A series of changes were seen in those rats treated for more than eight weeks. The changes included a mortality rate of 60%, decreased myocardial weight, low albumin levels, diminished ventricular motion, and left ventricular ejection fraction. Levels of triglycerides and cholesterol were elevated. The imaging studies showed a decrease in accumulation of both 18F-FDG and BMIPP [85]. Both experimental and clinical data have described low uptake of BMIPP following administration of adriamycin [84,86]. Loss of cardiac function due to doxorubicin has been described in cell culture using 99mTc-Sestamibi [87], a tracer that shows mitochondrial membrane potential. Takemura and Fujiwara published an experimental characterization of cardiotoxicity due to doxorubicin showing interstitial fibrosis, cardiomyocyte vacuolation, and fibrosis [88].
Inubushi evaluated this relation between fatty acid oxidation by BMIPP imaging and LEVF and showed a positive correlation between both methods [89]. It follows, that low LEVF values, which are often taken a cardiotoxicity marker, represent impaired cardiac free fatty acid utilization.
A functional relation between BMIPP and doxorubicin toxicity was described by Saito et al. in using a quantitative method for the determination of the k-value of tracer uptake [90]. The study group included 36 subjects with various malignancies treated with a mean dose of 257.8 mg/m² doxorubicin. The imaging procedures were done 7 to 28 days after treatment. Using this quantitative method, they described a harmful effect such that low k-values were evident (Figure 4) [90]. This parameter was found to be associated with a poor prognosis. Using this methodology, Saito et al., assessed fatty acid oxidation in connection with taxan chemotherapy and showed that cardiac fatty acid metabolism was diminished after chemotherapy while perfusion remained normal (Figure 5) [91]. BMIPP uptake was lower after chemotherapy and correlated with lower LVEF values (Figures 4 and 5) [91].
Figure 4: Significant increase in cardiac fluorodeoxyglucose uptake in post-adriamycin treatment (on right) fluorodeoxyglucose positron emission tomography scan compared to pre-treatment fluorodeoxyglucose positron emission tomography scan (on left).
Figure 5: Case example-LV SUVmax in Baseline (5.86), Interim (8.95/52.73% percentage increase from baseline) and Post-therapy PET/CT (9.67/65.02% percentage increase from baseline). LV: Left Ventricle; PET/CT: Positron emission tomography associated with computed tomography scans; SUV: Standard Uptake Value; SUVmax: Maximum SUV.
A clinical retrospective study published by Sarocchi et al. evaluated 43 patients with Hodgkin's lymphoma who had received doxorubicin. The final 18F-FDG PET imaging procedure was done almost two years after treatment. They found an increase of cardiac 18F-FDG uptake in doxorubicin-treated patients together with a decline in left ventricular ejection fraction [92]. In this research group proposed that an increased myocardial 18F-FDG uptake could be taken as a doxorubicin-induced oxidative stress marker [93].
In clinical settings, some treatment approaches combine chemotherapy with external radiation therapy. Billingham et al. described an enhancement effect of adriamycin cardiotoxicity when irradiation had been added [64]. An interesting fact concerning this effect was that irradiation showed this negative effect even when the treatment had been administered months to years before. The severity of these findings was confirmed by histopathology. Umezawa et al. [94] evaluated radiation-induced cardiotoxicity using BMIPP radionuclide imaging in patients who had received different irradiation doses for esophageal cancer. The original (Figure 4) from page 885 demonstrates the deleterious effect of external irradiation on the heart, i.e., disrupted fatty acid metabolism seen as a BMIPP uptake defect. The authors concluded that these images suggest myocardial damage. The loss of tracer uptake (red arrows) was described as being directly related to the administered radiation dose (Figure 2).
Besides these imaging results, previous studies from this research group had shown high myocardial 18F-FDG uptake following irradiation for esophageal cancer [95] together with significant elevation of BNP levels [96]. These results supported the diagnosis of cardiac damage with loss of fatty acid uptake, increased glycolysis, and an elevated level of cardiac damage marker. The experimental effect of external X-ray radiation with 20 Gy dose on heart metabolism of beagle dogs was investigated by Yan et al. [97]. 18F-FDG PET was done initially as well as three months later. They found high tracer uptake in the irradiated field and hypothesized that this was due to microvascular damage and mitochondrial injury. The results varied in intensity and were shown in a grading fashion (Figure 3) [97].
Anthracycline administration has been found to induce elevations of BNP. A poor prognosis of the disease was observed in patients with persistent elevations of BNP [98]. A persistent elevation of NT-proBNP following high dose chemotherapy for aggressive malignancies was associated with cardiac dysfunction [99]. Concerns about cardiotoxicity in the same setting have been reported by Zaucha-Prażmo et al. [100]. High-dose cyclophosphamide as part of BEAC treatment (BCNU, Etoposide, Cytarabine, Cyclophosphamide) can lead to acute subclinical systolic dysfunction, which was accompanied by an elevation of NT-proBNP levels. It is important to note that the patients in this study had elevated NT-proBNP levels, 445 ± 65 pmol/L, before therapy [101]. This fact suggests that cardiac impairment was already present in these patients. New data on the use of NT-proBNP concerning chemotherapy and cardiac dysfunction have shown the clinical validity to this marker [102-104].
Nuclear medicine diagnostic imaging of cardiotoxicity using 18F-FDG
Borde Kand, and Basu described the patterns of change of 18F-FDG uptake in lymphoma patients treated with adriamycin. Subgroup A of this study (n=8) showed an increased cardiac uptake (Figure 2)[105]. Their original Figure 1 image is reproduced here.
Several studies have documented cardiac uptake of 18F-FDG as seen in staging examinations [106-108]. Jingu et al., described increased 18F-FDG uptake after radiotherapy for oesophageal cancer (Figure 2) [109]. Specific description of cardiac uptake, however, was not discussed in these studies. Gorla et al., described a substantial increment of cardiac 18F-FDG uptake (Figure 2) [110] in a patient with Hodgkin’s lymphoma who had been treated according to an ABVD regimen. The authors suggested that this uptake represented cardiotoxicity after administration of adriamycin.
Matteo Bauckneht and coworkers have published a series of papers investigating the effects of doxorubicin on cardiac 18F-FDG metabolism since 2017 [92,93,111-113]. In the publication they concluded that this procedure was a biomarker for oxidative damage due to doxorubicin [93]. In his PhD thesis published [111] he added the following comment: “These results substantiate emerging literature putting attention on the effects of anthracyclines on myocardial retention of FDG, and raise research and realistic prospects. First, our and other authors’ work prompts the question of which mechanism underlies the higher accumulation of FDG in the myocardium exposed to DXR as compared with control conditions. Finding the answer would imply gaining insights into the pathogenesis of DXR cardiotoxicity, which is complicated and still to be fully resolved, and possibly laying the foundations for novel strategies to avoid or mitigate cardiac injury”.
Haider et al., discussed the importance of cardiac 18F-FDG uptake pattern in relation to risk stratification of cardiovascular disease [114]. They found a relation between focal tracer uptake and a higher risk for cardiac changes and lower LVEF values (Figure 4) [114].
Gherghe et al., conducted a prospective study on cardiotoxicity following HER2 inhibitor therapy evaluating radionuclide ventriculography and NT-proBNP as a biomarker revealing a negative correlation between both parameters (Figure 5) [115]. This association was found both in the baseline and in the follow-up examination. We interpret this finding as a sign for pre-existing cardiac disease.
Dourado et al., demonstrated increased 18F-FDG cardiac uptake after chemotherapy and interpreted the findings as a sign of cardiotoxicity (Figure 6) [116].
Figure 6: Administration of doxorubicin will cause a displacement of CoQ10 from mitochondrial respiratory Complex I leading to an acquired dysfunction.
In an accompanying editorial, Mesquita et al., stressed the importance of the findings in view of the association between diagnostic imaging and potential identification and definition of heart disease preventive interventions [117].
The spectrum of nuclear medicine methods for cardiac diagnostic imaging have been summarized by Cadour,Thuny, and Sourdon (Figure 2) includes methods for perfusion, mitochondrial, and metabolic imaging [118].
A recent review by Cannizzaro and coworkers, described current cardiac imaging methods for cardiotoxicity. While the authors recognized that cardiac metabolic dysfunction, e.g., myocardial perfusion and mitochondrial function, might be found in these patients [119]. They dismissed the clinical utility of these imaging methods [118].
An evaluation of cardiotoxicity due to anthracycline administration was published by Becker, Arruda, Berenguer, Buril, Cardinale, and Brandão [120]. Their analysis summarizes mechanisms of cardiotoxicity in Figure 2 including damage of mitochondria, accumulation of iron, inhibition of topoisomerase, induction of a proinflammatory state, and damage to sarcomeres. Although they recognized the importance of metabolic imaging, there was no treatment recommendation for these patients [120].
Our understanding of cardiotoxicity and heart failure is shown in Figure 5. The central issue is loss of mitochondrial function due to CoQ10 deficiency. In a series of publications, we have demonstrated both this pathogenetic principle as well as the treatment strategy for thyroid disease [121-125]. One key event related to CoQ10 deficiency is the development of hypoxia [126]. We have recently analyzed further biochemical changes of hypoxia stressing the role of CoQ10 deficiency [127].
Doxorubicin-induced mitochondrial damage and reduced cardiac function
Figure 6. Administration of doxorubicin will cause a displacement of CoQ10 from mitochondrial respiratory Complex I leading to an acquired dysfunction. Low level of ATP production will affect cardiac function which is reflected by diminished left ventricular ejection fraction. Nuclear medicine diagnostic imaging will show decreased fatty acid utilization in the BMIPP scan, and increased glycolysis in the 18F-FDG positron emission tomography. Clinical research data have shown a relation between deficient CD36 expression and low fatty acid utilization. CoQ10 supplementation can treat these defects.
Looking beyond oncology: Postpartum cardiomyopathy and cardiotoxicity the role of selenium and BMIPP
In recent years a new clinical entity has been associated with cardiotoxicity, i.e., postpartum cardiomyopathy. Davis and Brown described a woman who developed congestive heart failure postpartum. Seven years before this event, she had received doxorubicin because of osteosarcoma [128]. Newer case reports and risk evaluations have appeared since 2013 [129-132].
Descriptions of peripartum heart failure (PPCM) have been published for 170 years. Early reports were published by Ritchie [133] and by Porak in [134]. A review on PPCM published by Gouley in considered the disease to be idiopathic [135]. Hull [136,137] the term PPCM was used by Demakis and Rahimtoola [138]. Davidson et al. made a detailed description of cardiac failure in the perpartum as seen in Nigeria, mentioning the potential connection to heat stress [139]). “Peripatum cardiac failure (PPCF) is common in Zaria, in Northern Nigeria, bur has not been described elsewhere in Nigeria except in Ibadan. The geographic origin of a series of 224 patients with PPCF was studued in Zaria, and a servay of syndrome as seen in hospitals and physicians in northern state of Nigeria was carried out; information was also gathered from medical and nursing studients from various tribal groups in the same area. It was found that PPCF is common in that areas of Hausa majourity, mostly around Zaria and Malumfashi, where the postpatrum practices of taking hot baths, laying on a hot bed, and taking large amount of kanwa ( a lake- salt rich in sodium) are purused with great vigour. These costume may impose critical load on a valnearable myocardium, and it seems that tribe and tradition could well explain thew high incidence of PPCF around the Zaria”. A citation of Davidson is shown in the above and a similar source from Ezem et al. from [140]. “There is an ancient area in Northern Nigeria inhibited mainly by traditionally-oriented natives, mostly Hausas and Fulanis.
Women of these tribes traditionally deilver their babies at home under the care of midwives, using the hospitals only as a last resort. Since cold is through to carry puerperal illnesses, it is the practice among these people to initiate hot bath of mothers immediately after delivery. 2 major complications of the practice of the peripartal cardiac failure and burns as a result of the practice. The case is reported of 1 women who was taken to hospital with superficial burns as a result of practice. She had continued to follow this practice despite earlier experiences of the cardiac failure by doing so. It may be easier to persuade these natives to use slightly colder water than to give up completely this entrenched practice”.
As of, the pathogenesis of PPCM has not been explained. Jha and Jha reviewed current data on the disease and produced a tentative model of pathogenesis built around the production of reactive oxidative substances (ROS). The weak point of the model is that ROS production has not been explained (Figure 2)[141].
Cénac had described low selenium levels in PPCM patients [142-146]. Karaye reproduced these findings [147]. However, further biochemical dimensions of the results were not recognized. Situations where selenium levels are deficient correlate also with CoQ10 deficiency [148,149]. This indirect evidence would validate our model of cardiotoxicity in PPCM. CoQ10 has not been evaluated in PPCM.
Lwin et al. reported the results of 123I-BMIPP scintigraphy carried out in a woman who was referred because of hypertrophic cardiomyopathy while breastfeeding her child. While the authors described the uptake of the tracer in the breast of the lactating woman, other findings were not described. (Figure 3) clearly shows that the septum and the apex had uptake defects [150]. The fact that BMIPP uptake defects can be found in hypertrophic cardiomyopathy [151] supports our interpretation. As described in the preceding sections, low BMIPP uptake correlates with low LVEF, thus explaining heart failure. This has also been shown in a comparative study using BMIPP and tissue Doppler imaging such that BMIPP defects correlated with decreased ventricular function [152]. Conclusion the early diastolic MVG in the LV wall obtained by TDI patients with HCM correlates with myocardial fatty acid metabolism abnormalities detected by 123I-BMIPP myocardial scintigraphy. The MVG can be used as a marker of the severity of functional abnormalities in the regional LV myocardium in patients with HCM.
It shows the original conclusions of the study.
A disease found to be associated with PPCM is preeclampsia. Cardiac changes seen in these patients bear some similarity to those seen in cardiotoxicity [153-157]. Bello et al. proposed that both diseases have similar pathogenesis [153].
Additional biochemical information on preeclampsia relates to CoQ10. Enrique Teran and associates have described low levels of CoQ10 in eclampsia patients compared to normal pregnancies [158,159]. They proposed that mitochondrial dysfunction due to CoQ10 deficiency was the disease mechanism in eclampsia [160].
Connecting heat stress to cardiovascular disease and PPCM
The original setting of PPCM is related to heat exposure. The most salient evidence on the influence of heat stress on mitochondrial function comes from the known thermo-lability of Complex I and especially of CoQ10 [161]. Pobezhimova et al. have shown that under experimental conditions, heat stress affects the function of Complex I, the main change being its inactivation [162].
A similar result of altered function of Complex, I through heat exposure, has been reported by Ludwig et al., in a system that used beef heart mitochondria [163]. The isolated CoQ10 substance also shows lability to heat stress at 45° to 55°C [164]. A decrease in the levels of CoQ10 will alter and diminish the function of Complex I of OXPHOS. Mitochondria lacking CoQ10 will then produce more ROS. If this occurs in the heart, then heart function will be compromised. Ide et al. proposed that Complex I could be the source of ROS in the failing heart [165]. The heart is indeed the organ with the highest CoQ10 content in the body, and low levels can consequently affect cardiac function [166]. A similar situation can be expected in conditions such as cardiomyopathy [167].
Recently two publications have looked at the relationship between cardiac disease and heat stress. Ranek et al. described changes of heat shock proteins in heart failure, pointing towards the increased expression of hsp70, hsp90 and BAG-3 [168].
Iguchi et al. looked at cardiovascular and hormonal changes following heat stress produced by sitting in a heat stress chamber for 30 minutes at 73°C [169]. They demonstrated that this stimulus had an elevation of hsp72 and prolactin. The increase in prolactin levels seemed related to the endurance capacity in response to exercise under heat conditions [170]. Heat exposure and muscular activity can also affect blood magnesium levels inducing a severe decrease to suboptimal levels [171].
In a recent epidemiological analysis, Wang et al. described an association between previous heat stress situations and heart disease [172]. Nzvere et al. have reached similar conclusions in evaluating long-term consequences of heatstroke in reference to cardiac disease [173]. We propose that these authors were dealing with exposure to heatstroke which had led to a condition of CoQ10 deficiency. Unfortunately, this biochemical situation was neither investigated nor treated.
In short, the take-home message from this section can be stated as follows. Heat stress can lead to lower magnesium levels and elevation of prolactin. Heat stress can affect CoQ10, which will consequently alter and diminish the function of Complex I of OXPHOS. Loss of magnesium will affect the function of Complex V. Mitochondria lacking CoQ10 will then produce more ROS and less ATP. One can expect that inefficient ATP production in the mitochondria will be associated with fatigue as a key symptom. Combining magnesium, selenium, and CoQ10 will restore mitochondrial function and prevent ROS production.
CD36 deficiency is another mechanism that could explain cardiotoxicity and PPCM. A deficient state is associated with decreased BMIPP uptake. This defect can be corrected by administering CoQ10 [174-179].
Gonadotoxicity
The radiosensitivity of ovary to radiation has been known for decades. Medical history contains an account of methods to destroy ovarian function more than 100 years ago. The use of Roentgen rays for achieving castration was described as an auxiliary therapy for breast cancer [180]. The technique had been promoted and developed since 1889, when presented at a surgical congress. Following this same therapy concept, 181. Peck et al. presented data on female castration utilizing irradiation [181]. Brinkley et al. described the effects of deep X-rays for treating breast cancer in relation to menopause. The study included 267 women treated between 1942 and 1952 at Addenbrooke’s Hospital in Cambridge [182]. A follow-up evaluation done some 16 years later revealed increased mortality related to heart disease, cerebrovascular disease, and cancer at the heavily irradiated sites. The course of breast cancer was not influenced by this therapy[183]. Ovarian ablation through radiotherapy was still used in 1994 for breast cancer [184]. Effects of gonadal irradiation were described by Lushbaugh and Casarett [185]. In 1989 Wallace et al. described ovarian function changes in patients treated with whole abdominal external radiation. Treatments had been done between 1942 and 1985. The authors identified fifty-three patients for the evaluation and concluded a poor outlook for normal ovarian function after therapy [186]. Ovarian biopsies in ten patients treated for childhood leukemia were analyzed by Marcello et al. in 1990. In the small series of cases, they found a reduction in the number of follicles, ovarian fibrosis, and changes in the structure of blood capillaries [187].
Larsen et al. evaluated ovarian function in 100 cancer survivors in Denmark. The patients had received both chemotherapy as well as radiation as treatment modalities. The study found that one out of six patients developed premature ovarian failure. [188]. Chemaitilly et al. conducted a retrospective analysis of cancer survivors looking for the development of acute ovarian failure. A detailed analysis of the treatment modality and dose was carried out. Starting from 14372 patients included in the Childhood Cancer Survivor Study, the authors selected 3390 cases for the study. Inclusion criteria included age >18yr and having valid data on menstrual history. Acute ovarian failure was defined as cessation or absence of menstruation within five years after therapy. The affected patients were younger than 20 years at the time of diagnosis and 32.9 years of age at the study. The initial diagnoses included leukemia, lymphomas, Wilms’ tumor, neuroblastoma, soft- tissue sarcoma, and other entities. Treatment modalities are in [189]. Acute ovarian failure was found in 6.3% of cases.
Green et al., described gonadal dysfunction after chemotherapy given to cancer patients. The same group described gonadal changes after cancer treatment in children [190]. Similar observations were published by Whitehead et al. [191]. The authors commented that the mechanisms of ovarian changes were poorly understood.
The question of fertility and reproduction in young patients after therapy for cancer was addressed by Meirow [192]. Levine et al. have described the development of premature menopause in cancer survivors treated with either procarbazine or radiotherapy or who had had stem cell transplantation [193]. Recommendations for surveillance for cancer survivors due to potential risks for cardiomyopathy and premature ovarian failure have been published without and therapy suggestions [194-197] (Figures 6, and 7).
Figure 7: Biochemical adaptations to CoQ10 deficiency and hypoxia. IL-6 elevation is commonly mistaken as a sign for immunological activation and inflammation.
Based on the evidence presented we propose that the pathogenic mechanism of anthracycline cardiotoxicity is caused by CoQ10 deficiency following chemotherapy or radiation resulting in acquired mitochondrial dysfunction. A central metabolic event is the switch from fatty acid oxidation to glycolysis. Low level of fatty acids utilization correlates with diminished LVEF values which characterize heart failure. We believe that this same principle can explain other heart affections characterized by low values of left ventricular ejection fraction.
The visualization of glycolysis using 18F-FDG allows the interpretation hypoxia is a central element. In turn, hypoxia will induce a response that includes a series of biochemical changes.
We propose that clinical evaluation of oncological patients must include determinations of CoQ10 and NT-proBNP prior to therapy as well as during follow-up. Administration of CoQ10 has been shown to increase mitochondrial levels by which treatment of cardiotoxicity is feasible. This intervention finds applicability in heart disease. We have published further details on CoQ10 physiology and supplementation elsewhere.
Anthracycline administration will cause a displacement of CoQ10 from Complex I of the OXPHOS chain. Heart metabolism will switch from FAO to a hypoxic condition associated with glycolysis. Low utilization of FA correlates with low LVEF. Altered function of Complex I will lead to low ATP production, oxygen sensing can be compromised, ubiquinol levels can be low, ROS production increases. The main symptom is fatigue. Elevated NT-proBNP is a biomarker of the process. Failure to correct CoQ10 levels can end in heart failure.
Conceptualization, writing, editing RM and HM. Graphic work was done by RM.
This work received self-funding by Womed.
Not applicable.
Not applicable.
Data sharing not applicable.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar] [PubMed]
[Crossref][Google Scholar][PubMed]
[Crossref]
[Crossref][Google Scholar][PubMed]
Citation: Marcin R, Mewcy H. (2024) Identifying Hypoxia and Glycolysis in Chemotherapy- and Radiation-Induced Cardiotoxicity Based on Nuclear Medicine Diagnostic Imaging with 18F-Flurodeoxyglucose-Key Role of Coenzyme Q10 Deficiency In Acquired Mitochondrial Dysfunction. J Cancer Res Immunooncol. 10:195.
Received: 21-Feb-2024, Manuscript No. JCRIO-24-29702; Editor assigned: 23-Feb-2024, Pre QC No. JCRIO-24-29702 (PQ); Reviewed: 05-Mar-2024, QC No. JCRIO-24-29702; Revised: 15-Mar-2024, Manuscript No. JCRIO-24-29702 (R); Published: 22-Mar-2024 , DOI: 10.35248/2684-1266.24.10.195
Copyright: © 2024 Marcin R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work received self-funding by Womed.